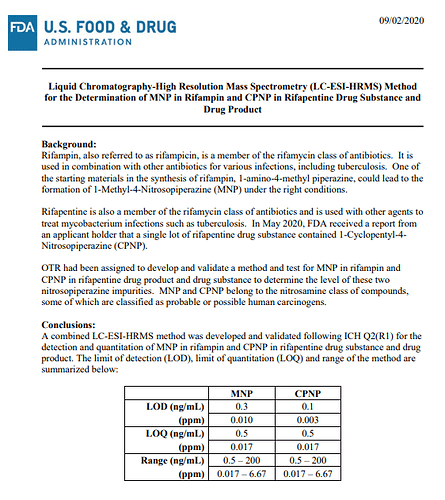
FDA-published testing method to provide an option for regulators and industry to detect nitrosamine impurities in rifampin and rifapentine drug substances and drug products. This method should be validated by the user if the resulting data are used to support a required quality assessment of the API or drug product, or if the results are used in a regulatory submission.
2 Likes