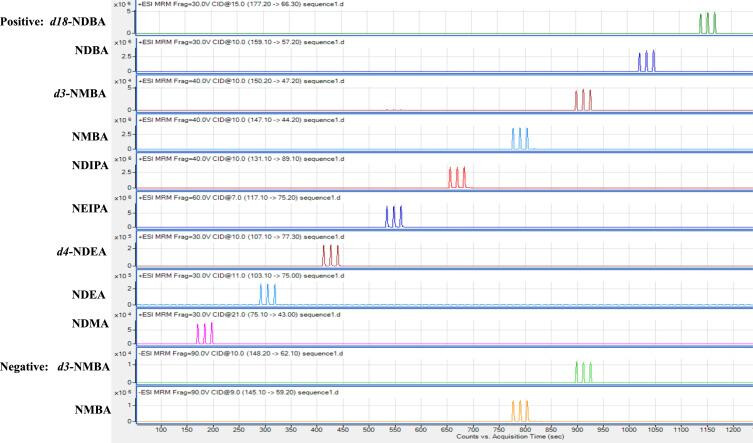
FDA laboratory developed and validated an automated micro-solid phase extraction MS/MS method, where all the analytes are not separated prior to elution to the MS, to simultaneously quantify NEIPA, NDIPA, NDBA and NMBA in ARB drug substances with an instrument sample analysis time of 12 seconds. The method was validated according to the ICH Q2(R1) guideline, and was determined to be specific, accurate, precise and linear over the corresponding nitrosamine analytical ranges. The method has been successfully implemented to quantitate the four nitrosamine impurities in 129 generic losartan, valsartan, olmesartan, irbesartan and telmisartan drug substance samples from 32 lots; and 32 losartan and valsartan drug product samples from 6 lots.
https://jpharmsci.org/article/S0022-3549(22)00568-8/
@trust_user_a @Nitrosamines_Analyzer @trust_user_c