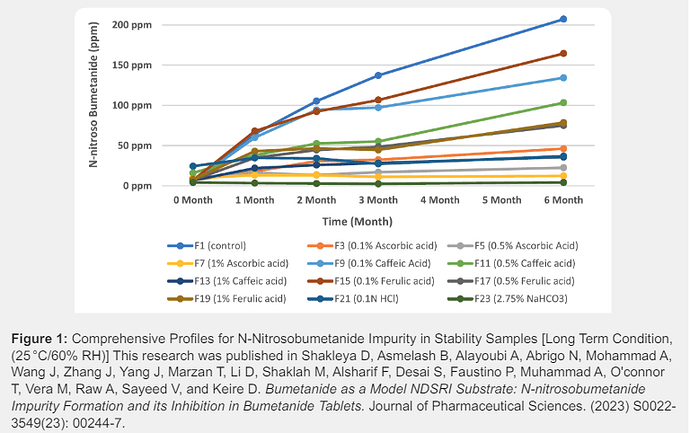
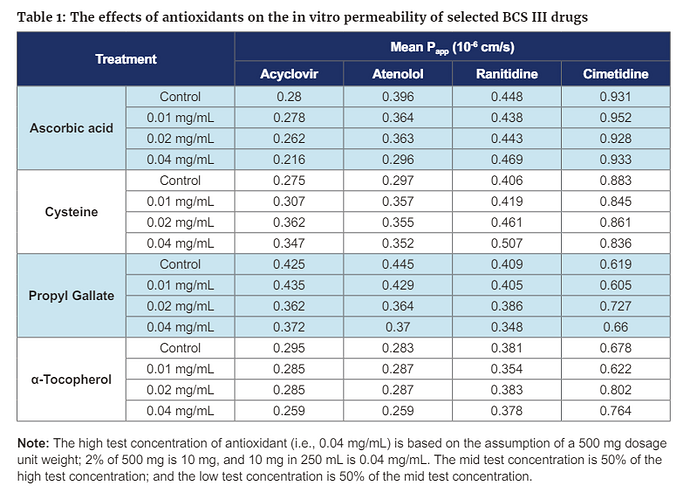
In FY 2023, research projects on impurities specifically focused on N-nitrosamines impurities including NDSRIs in drug products. FDA’s research efforts involved one external research contract and one external research grant, as well as many internal research projects related to nitrosamines impurities. These research projects continued to develop analytical procedures for the quantitation of N-nitrosamine impurities (small molecule nitrosamines and NDSRIs) in pharmaceuticals, assessing the risk of forming these impurities, toxicological risks of these impurities, exploring strategies to prevent or mitigate their formation by reformulating drug products potentially with suitable antioxidants or pH modifiers, and weighing the potential impacts of reformulations on the bioequivalence of generic products.
2 Likes