A notice says Nebivolol tablets are under recall because of a ‘nitrosamine drug substance related impurity.’ I think we’ve seen more and more news about nitrosamine-related recalls recently.
This page gives a good breakdown of some of the recalls that have happened this year, including the limits that have been exceeded (though not by what level) and the chemistry behind the synthesis of some of the APIs.
Growing Trend of Drug Recalls for NDSRIs – Qvents
A variety of different compounds, structures and nitrosamines formed - all look to be solid dose, some with extended release properties, and the associated methods of manufacture of the finished product.
Thank you, @MarkS. Chlorpromazine was recalled due to N-nitroso-desmethyl-chlorpromazine contamination, according to the same webpage.
I hope it will not be the beginning of the catastrophe.
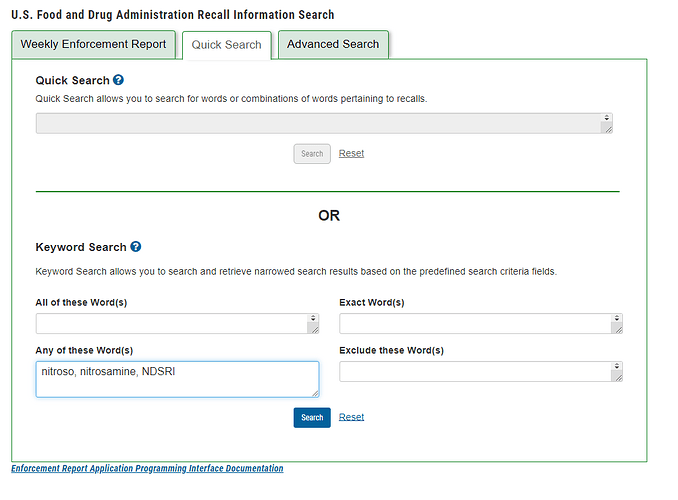
If someone wants to check the latest recall information in the USA, you can search in FDA Recall Information Search(Enforcement Report).
I recommend searching “nitroso, nitrosamine, NDSRI” in “Any of these word(s)” in Quick Search and exporting to CSV.