Disclaimer: The following summary represents my perspective on some relevant points discussed during the event “Mitigation Strategies for Nitrosamine Drug Substance Related Impurities: Quality and Bioequivalence Considerations for Generic Products” organized by the FDA and the Center for Research on Complex Generics.
1. Purpose: The workshop aimed to address the risks associated with the formation of Nitrosamine Drug Substance Related Impurities (NDSRIs) in certain drug products. It focused on discussing strategies to mitigate these risks and considerations for evaluating the safety risks associated with NDSRIs.
2. Approaches for N-nitrosamine Formation Risk: Model Amine Systems were utilized to study the potential formation of nitrosamines in oral solid dosage (OSD) products. These studies helped identify important risk factors such as:
- The vulnerability of amines based on pKa values,
- The addition of water during processing (wet granulation),
- The form of the amine (salt or free base), and
- The physical stability of vulnerable amines (crystalline vs. amorphous).
Further details on this topic can be found in: J Pharm Sci . 2023 May;112(5):1255-1267 (https://jpharmsci.org/article/S0022-3549(23)00028-X/fulltext).
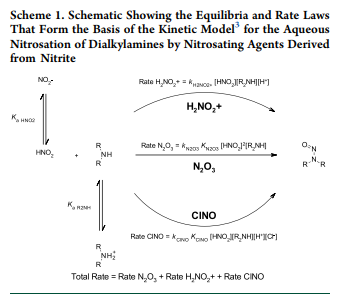
3. Reducing Nitrosamines Without Scavengers: A key assumption was made that the rate-limiting reagent in NDSRI formation is typically the trace levels of nitrite, rather than the more abundant amine in the active pharmaceutical ingredient (API):
- Selecting excipients with low nitrite levels (0.04 to 0.1 ppm) can reduce nitrosamine levels by up to tenfold compared to initial formulations. For most formulations, this approach effectively lowers the risk below the limit of 178 ng/day for NDSRIs;
- However, high-risk formulations may require additional mitigation strategies, such as transitioning from wet granulation to direct compression and incorporating nitrite scavengers to further reduce nitrosamines below the acceptable daily intake (ADI) limit of 18 ng/day.
4. Control Strategies for NDSRIs from Impurity Amines in APIs: The spatial location of nitrosation-susceptible amine impurities relative to the API can impact nitrosation kinetics. Controlling impurities in the final product is crucial in pharmaceutical manufacturing:
- Crystallization is a key unit operation used to reject and control impurities, enhancing purity throughout the synthesis process;
- The solubility-limited impurity purge (SLIP) test was employed to identify when an impurity forms its own pure solid phase during crystallization;
- Applying SLIP tests and similar approaches to APIs helps understand the microspatial distribution of impurity amine precursors, which can differentiate the risk level for NDSRIs when changing API sources or implementing new processes.
For more information, refer to Org. Process Res. Dev. 2023, 27, 723−741.
(https://pubs.acs.org/doi/10.1021/acs.oprd.3c00009)
5. Effectiveness of Antioxidants and Nitrite Scavengers: The effectiveness of antioxidants in mitigating NDSRI formation varied among selected model drugs:
- Ascorbic acid > caffeic acid > and ferulic acid demonstrated the highest inhibition of NDSRI formation among the antioxidants tested;
- The effectiveness of antioxidants depends on the drug substance, manufacturing, and formulation;
- Increasing the concentration of antioxidants improved NDSRI mitigation;
- Maintaining a neutral pH of the drug product served as a protective strategy against nitrosamine formation;
- The formation of NDSRIs was more pronounced under conditions of elevated heat and moisture during the drying step of wet granulation.
- Continuous manufacturing with direct compression may aid in nitrosamine mitigation.
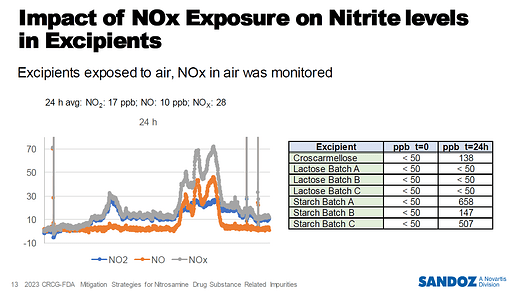
Additionally, the removal of nitrite, a key reagent for N-nitrosation, is considered a promising strategy:
- Nitrites can be present in water, APIs, excipients, and atmospheric NOx;
- PABA (para-aminobenzoic acid) was identified as an efficient inhibitor of N-nitrosamine formation in pharmaceutical dosage forms and showed comparable activity to ascorbic acid and l-cysteine;
- The selection of a nitrite scavenger should be carefully considered based on the amine structure, compatibility with the API and excipients, type of dosage form, and route of administration.
Additional information can be found in: Processes 2022, 10(11), 242.
(Processes | Free Full-Text | Assessment of a Diverse Array of Nitrite Scavengers in Solution and Solid State: A Study of Inhibitory Effect on the Formation of Alkyl-Aryl and Dialkyl N-Nitrosamine Derivatives)
Again, it’s important to note that not all points discussed during the workshop were included in the summary above.
I can explore those missed points in a future publication. The workshop covered a wide range of topics, including: additional mitigation strategies, analytical methods, regulatory considerations, and case studies, which deserve thorough attention.
What do you think?