Dr. Diab et.al published new paper related to the kinetic modeling of N-nitrosamine formation in aqueous solution with Di-n-butylamine. According to the paper, N -nitrosamine formation only becomes a significant risk in solution at pH < 6 and at higher concentrations of nitrite. Please read it!!
Abstract:
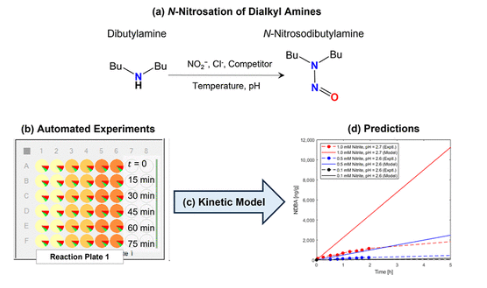
Healthcare marketing authorization holders are undertaking widespread risk assessment activities following the discovery of dialkyl N -nitrosamines in certain drug substances and products. A contribution to this exercise is the kinetic modeling of the reaction of secondary amines with nitrite ion in aqueous solution and identification of conditions where this would present a risk of N -nitrosamine formation. Herein, we describe automated experimental nitrosation studies on di-n -butylamine that highlight the conservative nature of the modeling approach and support the conclusion that N -nitrosamine formation only becomes a significant risk in solution at pH < 6 and at higher concentrations of nitrite. We report kinetic modeling with an updated treatment of temperature dependence and further experimentation on di-n -butylamine demonstrating our approach to be valid. Our experimentation extends to the consideration of N -nitrosamine formation from tertiary amines, which are considered less prone to nitrosation, as the mechanism includes a dealkylative step. We show a 2 orders of magnitude reduction in the rate of formation of N -nitrosodibutylamine from tributylamine compared with di-n -butylamine for the considered pH, nitrite, and amine concentrations.
Interesting findings:
-
This experimental work was set out to confirm the the aqueous solution behavior of different concentrations of secondary amine in the presence of variable nitrite concentration over a range of pH, keeping in mind the scenarios simulated by Ashworth et al. for nitrosation of N ,N -dimethylamine (DMA) -📚 OPRD Special Issue on Nitrosamines -Pub (Potential for the Formation of N-Nitrosamines during the Manufacture of Active Pharmaceutical Ingredients: An Assessment of the Risk Posed by Trace Nitrite in Water)
-
Analytical Challange: " Further investigation demonstrated that injection of samples containing amine and residual nitrite onto the chromatography column could increase the formation of NDBA leading to artificially high nitrosamine levels being detected. A proposal for this observation is that DBA is well retained by the column, while the nitrosating species in the sample are dissolved in the mildly acidic mobile phase and are able to react by flowing through the immobilized amine. It therefore became clear that quenching the nitrosation reagent would be required prior to analysis to obtain accurate quantification.
-
Effectiveness of quenching: “Except for aniline hydrochloride, we found the stability of the quenched samples to be unsuitable for kinetic analysis. In some cases, NDBA levels continued to increase postquench”
Related to the findings of the above article, two of the authors also just published