I have seen a post on LinkedIn of a copy of a letter that suggests the deadline for Stage 3 has been extended until 1st Aug 2028 for any nitrosamine that has had a limit published prior to 1st Aug 2025.
Has anyone reached out to Health Canada to get clarification on this? I know @Yosukemino reached to them before to inquire about another thing in the past.
The guidance will be updated soon.
1752253072968.pdf (112.6 KB)
August 1, 2025: An updated version of Health Canada’s Guidance on nitrosamine impurities in medications, including an updated list of established Acceptable Intake (AI) limits (Appendix 1), has been posted online. Several updates have been incorporated into the Nitrosamines guidance document, including:
- Health Canada’s current expectations for the Step 3 timeline of our Call for Review, notably:
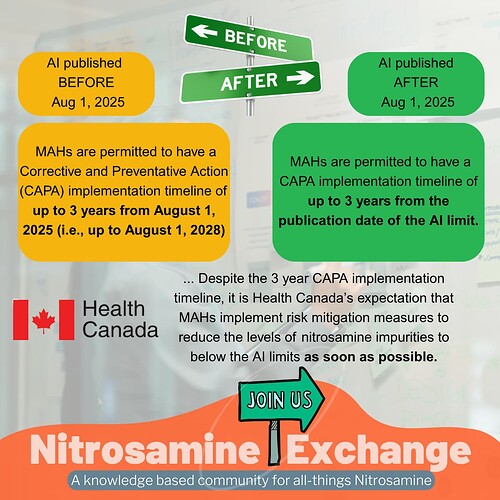
- For those nitrosamine impurities with an established AI limit published in Appendix 1 of this guidance document prior to August 1, 2025, MAHs are permitted to have a Corrective and Preventative Action (CAPA) implementation timeline of up to 3 years from August 1, 2025 (i.e., up to August 1, 2028).
- For those nitrosamine impurities with an established AI limit published in Appendix 1 on August 1, 2025 or later, MAHs are permitted to have a CAPA implementation timeline of up to 3 years from the publication date of the AI limit.
- Considering the risk profiles of nitrosamines and the possibility of an additive biological effect, the AI limits published in Appendix 1 are considered appropriate by Health Canada for lifetime and less-than-life (LTL) administration of a drug product, including during the 3 year CAPA implementation timeline (i.e., LTL-based AI limits are not to be applied during or after 3 year CAPA implementation timeline).
- General and Quality:
- Confirmatory testing expectations for nitrosamine impurities which can be controlled according to the ICH Q3A and ICH Q3B guidelines (number 12)
- Additional guidance concerning Supplements, Notifiable Changes and Post-DIN Change submissions where the quality changes do not increase the risk of presence of nitrosamine impurities relative to the approved drug product (number 20 and Appendix 2)
- Validating the limit of quantitation for analytical procedures (number 33)
- The potential for alternative control strategies where the root cause of nitrosamine presence is well understood (number 34)
- Appendix 1:
- seventeen (17) additional nitrosamine impurities and their corresponding CPCA-derived AI limits
- revised AI limit for N-nitroso-N-desmethyl-doxylamine (from 18 ng/day to 100 ng/day)
- The list of established AI limits can be filtered by date to identify the most recent additions
- Appendix 5:
- A new appendix concerning bioequivalence studies for reformulated drug products with nitrosamine suppressing agents such as antioxidants and pH modifiers
Appendix 5: Bioequivalence studies for reformulated drug products (new)
In general, qualitative changes to a drug product should be supported by a demonstration of bioequivalence of the reformulated product in comparison to the unmodified formulation, or to the Canadian reference product (CRP) for subsequent-entry (generic) drug products, in an in vivo comparative bioavailability study.
Reformulated immediate-release, solid oral or oral suspension dosage forms that contain Biopharmaceutics Classification System (BCS) Class I (high solubility, high permeability) drug substances are eligible for a waiver of the requirement to provide a bioequivalence study (biowaiver), per the recommendations detailed in the ICH M9 harmonised guideline Biopharmaceutics Classification System-based biowaivers. Additionally, a biowaiver may be considered for drug products with BCS Class III (high solubility, low permeability) drug substances that are reformulated to include specific antioxidants (i.e., ascorbic acid, α-tocopherol, propyl gallate, or cysteine hydrochloride). Recent studies have demonstrated that the incorporation of small quantities of these antioxidants in BCS III model drugs do not alter the permeability of the drug substance or the activity of intestinal transporters.Footnote1 Footnote2 Consequently, the addition of these specific antioxidants is not expected to impact the absorption or bioavailability of the drug. The results of these studies and conclusions can be extrapolated to drug substances that exhibit high permeability, given the minimal effect on the BCS Class III (low permeability) model drug substances. Therefore, a biowaiver may also be considered for drug products with BCS Class II (low solubility, high permeability) drug substances that are reformulated to include these specific antioxidants (i.e., ascorbic acid, α-tocopherol, propyl gallate, or cysteine hydrochloride).
As previously noted, a pH modifier (for example, sodium carbonate) that alters the gastrointestinal microenvironment to neutral or basic pH, may be employed to inhibit nitrosamine impurity formation. The addition of a pH modifier would not be expected to impact the permeability of a drug substance with amine functional groups. As such, biowaiver requests may be considered for drug products that contain BCS Class I, II, or III drug substances, that are reformulated to add a pH modifier.
A biowaiver for a reformulated drug product should be supported by a scientific justification of the choice and amount of the antioxidant or pH modifier employed in the formulation. In addition, the manufacturing process should remain generally unchanged apart from the addition of the new excipient.
The ICH M9 guideline provides recommendations to support the biopharmaceutics classification of drug substances and details regarding the criteria and data requirements for a biowaiver based on BCS principles.
Biowaiver requests for reformulated immediate-release, solid oral or oral suspension dosage forms that contain BCS Class IV (low solubility, low permeability) drug substances and reformulated modified-release products should be supported by robust approaches, such as a validated in vitro-in vivo correlation (IVIVC) or physiologically based biopharmaceutics modeling (PBBM). Biowaivers may be considered on a case-by-case basis for reformulated enteric-coated drug products that contain BCS Class I, II, or III drug substances when scientifically justified. If a biowaiver is not applicable, comparative bioavailability studies should be provided to support the change in formulation.
The Health Canada guidance documents, Conduct and Analysis of Comparative Bioavailability Studies and Comparative Bioavailability Standards: Formulations Used for Systemic Effects, should be consulted for study design and statistical analysis considerations, and for recommendations on the standards that should be met to determine bioequivalence, respectively.
Appendix 1: Established acceptable intake (AI) limits for N -nitrosamine impurities
17 Additional nitrosamines and one nitrosamine with revised AI are as follows;
| Nitrosamine | DP | CPCA | AI(ng/day) |
|---|---|---|---|
| N-nitroso-desmethyl-almotriptan | Almotriptan | 1 | 18 |
| N-nitroso-N-desethyl-amiodarone | Amiodarone | 1 | 18 |
| N-nitroso-N-desmethyl-cabergoline | Cabergoline | 1 | 18 |
| N-nitroso-N-desmethyl-diltiazem | Diltiazem | 1 | 18 |
| N-nitroso-N-despropyl-ropinirole | Ropinirole | 1 | 18 |
| N-((2- isopropylthiazol-4-yl) methyl)-N-methylnitrous amide (NNTA) | Cobicistat | 1 | 18 |
| N-nitroso-brinzolamide | Brinzolamide | 2 | 100 |
| N-nitroso-carvedilol | Carvedilol | 3 | 400 |
| N-nitroso-safinamide | Safinamide | 3 | 400 |
| N-nitroso-N-desmethyl-methadone | Methadone | 3 | 400 |
| N-nitroso-dalbavancin | Dalbavancin | 4 | 1500 |
| N-nitroso-norfloxacin | Norfloxacin | 4 | 1500 |
| N-nitroso-vancomycin | Vancomycin | 4 | 1500 |
| (2S,4R)-N-(6-bromopyridin-2-yl)-4-fluoro-1-nitrosopyrrolidine-2-carboxamide | Danicopan | 4 | 1500 |
| N-nitroso-vanzacaftor ^ | Vanzacaftor | 4 | 1500 |
| N-nitroso-acarbose | Acarbose | 5 | 1500 |
| N-nitroso-nadolol | Nadolol | 5 | 1500 |
| N-nitroso-N-desmethyl-doxylamine | Doxylamine | - | 100 (revised from 18) |
^ CPCA category was established using a correction to account for the 5-carbon chain feature length