Less-than-lifetime approaches and continued evaluation of the AI-value impact of (de)activating features remains very welcome, but don’t explain the removal of the AI from the list. It is indeed an advantage that lidocaine metabolism is very well described.
The mutagenicity of 2,6-xylidine has been significantly derisked in A weight of evidence assessment of the genotoxicity of 2,6-xylidine based on new and existing data.
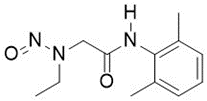
Metabolisation of lidocaine does not only involve 2,6-xylidine as a metabolite, but also monoethylglycinexylidide = desethyl-lidocaine, which is also a Ph. Eur. impurity (impurity D). Considering the high diversity in lidocaine products, from anesthetics for hospital use to a high diversity of OTC galenic forms, and desethyl-lidocaine being an known and intrinsically nitrosatable impurity, I do not find it improbable a product might exist that requires setting an acceptable intake (daily lifetime as point of departure of LTL AI design), as reflected in the limit list of EMA, HC and TGA, even if it is very likely that for many products this is not a likely nitrosamine as well. Recent stoichiometric studies show that nitrosation conversion and selectivity (C-nitration on aromatic ring vs. N-nitrosation, cf. structure of desethyl-lidocaine: C-nitration risk para of the amino-group of the 2,6-xylidine) might significantly differ liquid phase vs. solid phase. Though these studies are stoichiometric and under stirring/mechanical impact (possibly influencing extrapolability), general improbability of a synthesisable nitrosamine cannot be concluded when a high diversity of products exists. Many nitrosamines in the international limit lists cannot be formed selectively in stoichiometric liquid phase experiments but this does not per definition mean improbability of the impurity.
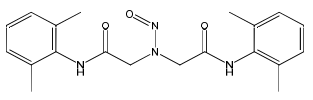
It is true that lidocaine EP impurity E has a double risk for C-nitration compared to lidocaine EP impurity D due to having two aromatic rings, but I deem the risk for mischaracterisation of the “nitrosamine” that was possibly reported reasonably low from a compound characterisation science perspective, making the scenario where the error lies in the redacting of the limit list more likely maybe (e.g. identified need based on step 2 submissions to list nitroso impurity D, but listing nitroso impurity E instead by reading the wrong line in the monograph).
When considering some elements of lidocaine/monoethylglycinexylidide metabolism extrapolatable to N-nitroso-monoethylglycinexylidide (N-nitroso-desethyl-lidocaine): I believe amidolysis metabolisation for beta-amido nitrosamines is intrinsically part of the design of the nitrosamine deactivating feature beta-amido (thus linking it with deactivating feature COOH: strong binding to plasma proteins and rapid excretion). The fact that the amine metabolite 2,6-xylidine is not highly potent supports the validity of this design (and often the theoretic amidolysis metabolite of the nitrosamine and the API will be the same), thus confirming that the nitrosamine and not the amine is the typical focus. (Comparable to how for nitrosamine metabolisation also the diazonium-ion related risk and not the formaldehyde risk is the focus based on potency ranking. For discussions of formaldehyde as an alpha-hydroxy-nitrosamine metabolite, including a discussion on the toxicity of formaldehyde: Peterson, L. A., Urban, A. M., Vu, C. C., Cummings, M. E., Brown, L. C., Warmka, J. K., Li, L., Wattenberg, E. V., Patel, Y., Stram, D. O., & Pegg, A. E. (2013). Role of Aldehydes in the Toxic and Mutagenic Effects of Nitrosamines. Chemical Research in Toxicology, 26(10), 1464–1473. https://doi.org/10.1021/tx400196j.)