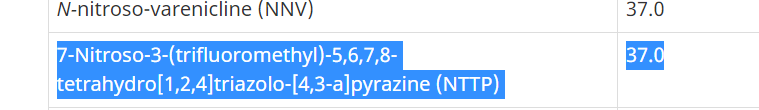
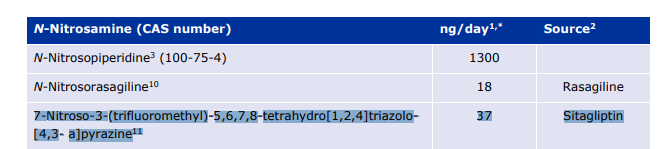
The Health Sciences Authority would like to update healthcare professionals on a newly discovered nitrosamine impurity, Nitroso-STG-19 (also known as NTTP) in sitagliptin products. Trace amounts of NTTP that were higher than the internationally acceptable limit was detected in only certain samples of sitagliptin products. The risk of taking sitagliptin products containing trace amounts of NTTP is very low as the potential risk of nitrosamines is associated with long-term use. The company will be making the necessary changes in the manufacturing processes to eliminate or reduce the amount of the impurity to acceptable levels. In the interim period, HSA is allowing a higher limit of NTTP in sitagliptin products so that patients can have continued access to these products. Healthcare professionals can continue to prescribe sitagliptin products and patients currently taking sitagliptin can also continue to take the medicine where clinically appropriate.
Please refer to the attached safety update that has been published on the HSA website (https://www.hsa.gov.sg/announcements/safety-alert/update-on-nitrosamine-impurity-in-sitagliptin-products) for further details on this issue.