Dear all,
How would you consider this structure below (2-Methylimidazole):
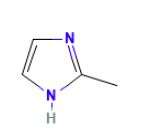
Could it be considered as a secondary amine source? or would it be outside of the scope of nitrosamine guidelines?
Thanks in advance!
Victoria
Dear all,
How would you consider this structure below (2-Methylimidazole):

Could it be considered as a secondary amine source? or would it be outside of the scope of nitrosamine guidelines?
Thanks in advance!
Victoria
Both N atoms are part of the aromatic system. The nitrogen in the NH group is non basic as the lone pair electrons are part of the resonance structure. It is known that for a non basic nitrogen it is difficult or not possible to form a nitrosamine and if it does, it cannot have the same carcinogenic mechanism as typical nitrosamines, based on alfa-hydroxylation and C-N cleavage as this would require breaking the aromaticity.
Check out this reference:
Great explanation, it seemed to me that it would not be possible to form nitrosamine, but now is more clear for me.
Thanks for the article!
Victoria
Please just consider that as per EMA guideline at least, you will need actual data indicating it was not possible to synthetize an specific nitrosamine if after your risk assesment the nitrosamine is realistically expected or nor enough evidence to dismiss the risk.
Thanks so much! The corresponding N-nitrosocompound is commercially available but as mentioned before, it is not a real N-nitrosamine structure. Thanks!!!
I believe @David can explained further the details of why we did not included in our assessment of the landscape publication. A good reference is also the adobo et al publication
I can indeed - as a basic secondary amine, a nitrosated imidazole can be formed. However, it’s nigh-on impossible for the ultimate genotoxic diazonium to form, since that would require destruction of the aromatic system.
Nitrosated pyrrole-type nitrogens can be mutagenic, but the mechanism is completely different - they are reduced (possibly via bacterial nitroreductases) to the aromatic hydroxylamine; an intermediate that may be familiar from the aromatic amine context. This then generates a nitrenium ion which is may be genotoxic, depending on substitution. This is the same mechanism as aromatic C-nitroso compounds can undergo, so in practical terms the N vs C nature of the nitrosated atom is less important than the fact it is aromatic, as we would perhaps expect. This different mechanism means that they are not expected to be of cohort-of-concern level potency, if positive.
We discuss this further - with a figure - in the recent OPRD paper here: https://pubs.acs.org/doi/10.1021/acs.oprd.3c00008
I am joining late in this conversation. There were too many things going on this week. I have had several quesitons with drugs like Etomidate, DEXMEDETOMIDINE and numerous others, which have imidazole moieties. Nitroso imidazoles form easily and some of them are potent carcinogens, especially amino imidazoles. Amino imidazoles are formed when we cook meat at high temperature. But they do not act by alpha hydroxylation though. In my opinion, we need to draw a line. Nitroso imidazoles are part the “cohort” but they may be given a lower priority in the current situation as the guidance documents talk of nitrosamines, and this is not a “nitrosamine”.
Thanks for your brilliant explanation!
I’ll try to access the article!
So as a summary, a nitrosated imidazole (which is not a not a real nitrosamine) can be formed. However, the mechanisch of mutagenicty and the “cohort-of-concern” potency would be different (lower for potency) for this Nitrosated imidazole when compared with a tipical nitrosamine structure.
Thanks again for your feedback!
Victoria
Thanks for your feedback! Agree, Nitroso imidazoles are within the “cohort” but not in the first place… ![]()
Dear David and ASrinivasan,
first of all thanks a lot for your scientific based answers (as usual).
allow me please to come back in this interesting topic.
So, given the fact that N-nitrosoimidazoles are mutagenic/carcinogenic then under which guideline should their specifications be set?
Logically we should use ICHM7 and a limit of 1500mcg/day. Is this correct?
I am asking this, because the usual approach when we prove that a nitrosamine is not CoC is to ‘‘move’’ its analysis to ICHQ3 guideline.
thanks for your time
Pseudonitrosamines are considered outside of the call for review, but not per definition outside of ICH M7(R2) CoC. The CoC in ICH M7(R2) is “N-nitroso-”, not nitrosamine.
The point of departure is governance under ICH M7(R2) but under the assumption that “even below the TTC of 1500 ng/day they would theoretically be associated with a potential for a significant carcinogenic risk.” So that theoretical assumption has to be evaluated against data in the development of the proposed AI.
Given the potential risk for an alternate mechanism with these, but a lack of the potential diazonium formation - both as described in the paper cited earlier - I would start with M7 (R2) indeed, and end up at 1500 as follows:
In the long term it is my hope that the M7 EWG, if and when they reconvene, take note of the paper linked above and redefine the cohort as described in it, to be effectively H-C-N-N=O (or nitrosamides etc)
Dear C. Wybon,
thanx a lot for your fast response.
If i understand well, an acceptance intake for a nitroso-imidazole compound, for which no known mutagenicity data is available, should be set as 1500mcg/day or less.
So, we are in the section 7.5 of the guideline, where is written:
‘‘Although the principles of this guideline can be used, a case-by-case approach using e.g., carcinogenicity data from closely related structures, if available, should usually be developed to justify acceptable intakes for pharmaceutical development and marketed products.’’.
Please dont call anything as “pseudo nitrosamines”, they are N-nitroso compounds. Nitrosamines are just a subclass of N-nitroso compounds. There are numerous studies on N-nitroso compounds, so you can also do a surrogate study to justify a higher than 1.5 mcg/day limit. Of course, a simple general Ames Assay will be a good option to control these using ICH Q3A and ICH Q3B.
Personally the fact that there are so many types of N-nitroso compounds that are not nitrosamines is why I like that somebody invented the term pseudonitrosamines.
The term pseudonitrosamines to mention everything N-nitroso (and thus initially CoC) that is not a nitrosamine (and thus out of the call for review (and CPCA for that matter)) I find quite elegant.
Pseudonitrosamine does not suggest non-nitroso, non-CoC or non-applicability of ICH M7 principles in its name, it says “not nitrosamine” not “not nitroso”.
What’s in a name? But these class of N-nitroso products are well known for many, many years. They are not “pseudo” anything. They are nitrosamides, nitrosourea, nitrosocarbamate, and so on. They have names based on IUPAC. The organic chemist in me does not want them to be called pseudonitrosamines. But I understand that in the realm of regulation, people need easy solution and differentiation at this time and I guess, again, whatever is a good differentiator would help.