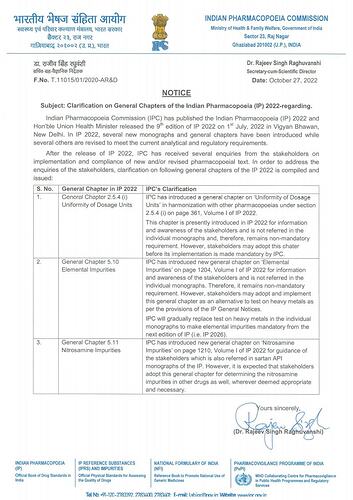
Indian Pharmacopoeia Commission (IPC) has introduced (a) new general chapter on “Elemental Impurities” in Indian Pharmacopoeia (IP) 2022, which will be mandatory requirement from the next edition of IP (i.e. IP 2026) (b) new general chapter on “Nitrosamine Impurities” for the guidance of stakeholders. It is expected to be adopted by stakeholders wherever deemed appropriate and necessary in all drugs testing.
https://www.ipc.gov.in/news-highlights/1051-ipc-s-notice-regarding-general-chapters-of-ip-2022.html
3 Likes
Good to know India’s stand on the nitrosamines. Although not formed a mandatory requirement considering its major impact on the domestic supplies and SME manufacturer.
2 Likes
Just wondering if there is any current enforcement for the national industry?
1 Like
Agree…at current stage this will be burdensome for SME and local mfg, but sooner or later it will be required. So if situation allows, mfg should make an effort to comply their products with regulatory requirements voluntarily. In long term, it will help to increase product quality and patients safety.
In recent past (May 2022), regulatory authorities asked mfg to perform nitrosamine testing. But limited analytical testing capabilities and cost aspects acted as big barriers for mfg specifically small and medium mfg selling products locally. Even many state regulators laboratories currently lack required analytical capabilities. But nitrosamine impurities inclusion for sartan APIs monographs and voluntary for other drugs in Indian Pharmacopoeia 2022, regulators hinted for eventual compliance requirements in coming years for indian maket.
1 Like