Last week (Oct 8th) Japan’s Drug Evaluation and Control Division, Pharmaceutical Affairs, Ministry of Health, Labour and Welfare posted a notification “Voluntary Inspection on the Risk of Nitrosamines Contamination in Pharmaceutical Products”
The intention is to instruct the manufacturers and distributors under your jurisdiction to conduct self-inspections on the risk of contamination with nitrosamines, in cooperation with manufacturers involved in the manufacture or packaging of APIs or preparations, suppliers of additives, reagents, and container closure systems, and the API’s National Administrator.
In order to facilitate this self-inspection, a separate question and answer document (Q&A) will be prepared, and an administrative notice will be issued shortly.
Eligeble drugs includes:
- Chemically synthesized ethical drugs, drugs requiring medical advice and OTC drugs.
- Biologics with high risk of contamination with the following nitrosamines:
- Biologics that contain chemically synthesized fragments and have risk factors equivalent to those of chemically synthesized active ingredients.
- Manufactured using a process of intentional addition of a nitrosating reagent
- Packaged using specific primary packaging materials (such as blister packs containing nitrocellulose)
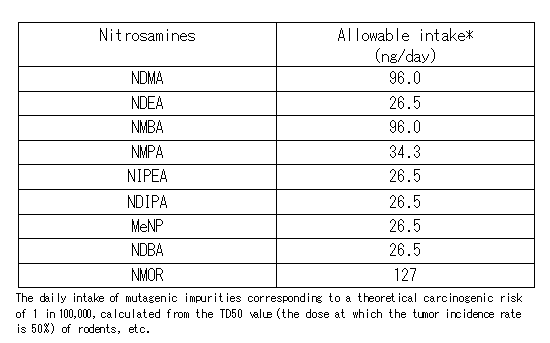
The limits for nitrosamines in pharmaceutical products:
Guidance document here: IDRAC_336737_10-Oct-2021_Notification_ PSEHB_PED No. 1008_1, PSEHB_PSD No. 1008_1, PSEHB_CND No. 100.pdf (160.3 KB)
