Dear Team,
May i have your assistance.
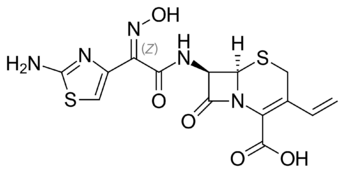
I am currently working on risk assessment for the presence of nitrosamine impurities in Cefdinir, the chemical structure of the API contains secondary and tertiary amines. However the API not mentioned in the EMA or USFDA list for NDSRIs. why is it not mentioned , is that mean no nitrosamine can be formed. could you please provide me with scientific answer to justify the case .
I dont see any secondary/tertiary amine on the structure that could be a potent amine precursor…
2 Likes
This issue is a little more complex and a deeper discussion is necessary.
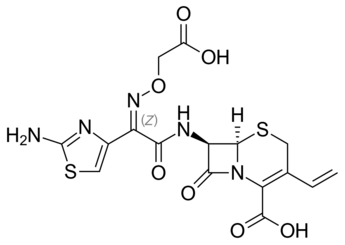
We made a deep risk assessment on Cefixime, which is very similar to Cefdinir:
Some vendors of analytical standard proposed the following potential nitrosamines.
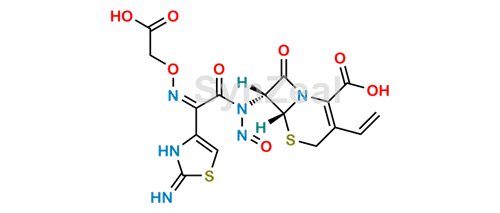
This one may be theoretically formed by the nitrosation of the amide nitrogen:
Cefixime Nitroso Impurity 1 (for more precision, thisone is a nitrosamide, not a nitrosamine).
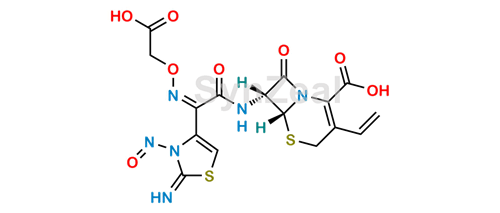
The second one by the nitrosation of the thiazole ring (much improbable, because the precursor should be a non-aromatic tautomer).
Cefixime Nitroso Impurity 2
However, any verdor claims to have these impurities in stock, so, in my opinion, no one has synthesized them yet.
In our opinion, none of the above theoretical impurities may occur in the reality, because the primary amino group is much more reactive and practically acts as a nitrite scavenger.
The reaction between the primary amino group of Cefixime and nitrites has been confirmed by the literature and it was used in the following analytical method:
Spectrophotometric determination of nitrite in soil and water using cefixime and central composite design
Therefore, we concluded that any nitrosamine and/or nitrosamide can occur in Cefixime.
Considering that the structure of Cefdinir is practically the same, also in Cefdinir any nitrosamine and/or nitrosamide may be formed.
7 Likes
Thank you Paliog for the valuable comment.