The Korean National Institute of Food and Drug Safety Evaluation published this report this week.
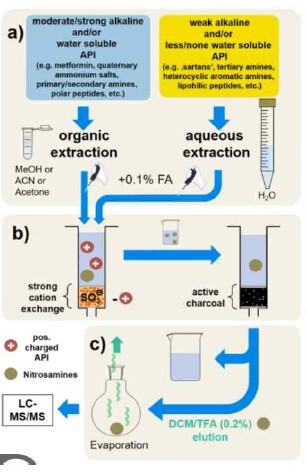
Pay special attention to their results on the formation of NDMA under manufacturing conditions such as: Water content during granulation, drying temperature, and granule size. The use of activated-carbon as a process mitigation and during sample preparation tool.
This casebook analyzes the causes of NDMA formation during the synthesis, formulation, and storage stages of pharmaceuticals and summarizes various reduction methods. It is expected to help in the design of product development for pharmaceuticals with potential nitrosamine impurity formation.
In the synthesis stage, the main causes of nitrosamine impurities in valsartan are identified, and methods to reduce them are explained. The formulation stage examines the causes of NDMA formation during the metformin formulation process. It highlights differences in NDMA levels between immediate-release and sustained-release formulations and identifies key factors influencing NDMA formation. The storage stage focuses on nizatidine and the potential for NDMA formation during storage. It evaluates the effectiveness of activated carbon and silica gel as adsorbents to reduce NDMA levels. The placement of adsorbents within the packaging is analyzed to determine the optimal configuration for minimizing NDMA formation.
The contents are as follows:
I. Introduction
II. Mechanism of Nitrosamine Formation (General Conditions)
III. Synthesis Stage: Reducing NDMA in Valsartan Synthesis
IV. Formulation Stage: Reducing NDMA in Metformin Formulation
V. Storage Stage: Reducing NDMA During Nizatidine Storage
VI. Discussion
Korean Version:
IDRAC_406286_01-May-2025_NIFDS Guide-11-1471057-100007-01_ Case Studies on Reducing Impurities in Ph.pdf (1.7 MB)
English version (Google translated):
Machine Translated Document - IDRAC 406286.pdf (9.3 MB)