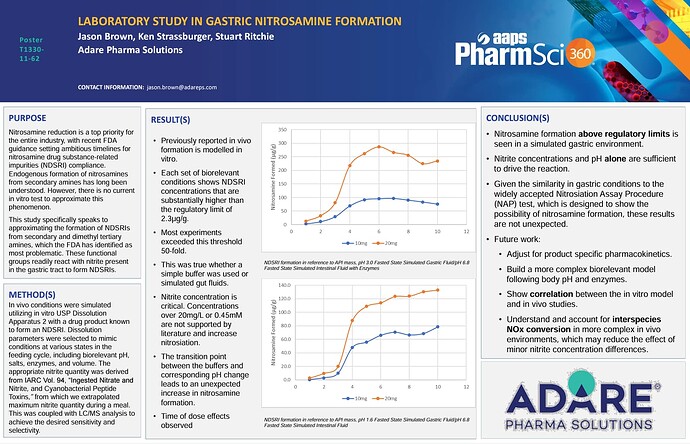
See our attached poster from the AAPS conference. Please follow up with any questions. Our findings support previously seen in vivo nitrosamine formation. The extent of formation is astounding & points to a potentially larger patient safety concern than what is seen in drug product directly.
LABORATORY STUDY IN GASTRIC NITROSAMINE FORMATION Poster.pdf (448.3 KB)
Hi Jason. It is really valuable information
Very nice work Jason,
just a question
the x axis is the time of the nitrosation reaction?
I am not sure if the results point to a larger safety concern than to a more accurate and more realistic acceptance intakes for the NDSRIs and for nitrosamines in general.
best regards
Christos
Correct. The x axis is time in hours.
Our conclusion was the same as you point out. It speaks both to larger safety concerns and that the limits should be considered in this context.
Hello everyone! I’m wondering whether the tendency of an API to form a specific nitrosamine compound in the stomach could serve as a basis for relaxing the impurity limit for that nitrosamine, especially when there is ample real-world data demonstrating the drug’s safety. If not, why?
hello.
if there is adequate specific experimental data (carcinogenicity, TD50, safety) for your nitrosamine/ nitrosocompound, then you can support a higher limit.
Otherwise, although this assumption is pretty much the logical one, it is not of regulatory acceptance.
Hi @daixulin,
It may be acceptable if you say the impurity is under ICH M7. However, the regulations for nitrosamines are different from those for ICH M7. I agree with Eleni. At least for now, such methods are not approved by regulators.
As the EMA is cooperating with the following studies, further discussions may take place in the future.
https://doi.org/10.1002/dta.3874
Right. This is our understanding as well. The thought and theory is supported by the guidelines, but in application for nitrosamines, it is not accepted.