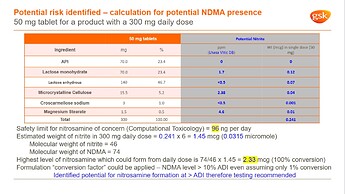
I recently came across this report from a Lactose manufacturer "Conclusion: Neither the chemical composition nor the processing conditions indicate any possibility for nitrosamine contamination and formation in the process or storage of the MEGGLE product"
I’m curious what has been the experience for our members working with excipient manufacturers. Is that enough for your risk assessment? Are you getting any pushback from Regulatory bodies?
Lactose Monohydrate USA, Nitrosamines.pdf (314.2 KB)
We have had similar responses from a number of manufacturers. Unfortunately it does not address the possibility of nitrite which is the bigger question in most cases.
1 Like
@Naiffer_Host @GENERAPHARM
It’s a good example. Though I have no experience of getting any pushback from Regulatory, I think it’s enough for risk assessment because it includes all of necessary information on the latest IPEC questionnaire. For API without nitrosable amine structures, it does not cause nitrosamine contamination risk in general. For API with nitrosable amine structures, I’m not sure which is better for the amount of nitrosating agents in this excipient, 0.39 ppm, 0 ppm or 3 ppm, however I think 0 ppm can be assigned because it is natural origin and without nitrogen, excipient with the lowest risk of nitrosating agents contamination. What do you think?
Lactose almost certainly contains nitrate/nitrite so zero is just undefendable
1 Like
@Yosukemino @GENERAPHARM I am really curious how organizations are striking for that balance, excipients manufacturing - DP manufacturers.
From the recent Inudstry-FDA nitrosamine meeting, FDA was quite clear in their response:
“Drug product manufacturers need to verify that the excipients used in their process and in conjunction with other ingredients, including the active ingredient and any processing aids, do not lead to the formation of objectionable nitrosamine levels.”
2 Likes
I share the good example of nitrite calculation contained in excipients and others demonstrated by Dr. Urquhart in nitrosamine workshop last week.
According to him, contamination risk is evaluated with 100% conversion at first. Next a conversion factor, whose detail is not included here, is considered and the calculated value is compared with (adjusted) ADI. This example may concern with API with secondary or tertiary amine, however if the amount of these amines is at impurity levels, the contamination risk of nitrosamines is not crucial in general, as illustrated in the paper, Potential for the Formation of N-Nitrosamines during the Manufacture of Active Pharmaceutical Ingredients: An Assessment of the Risk Posed by Trace Nitrite in Water.
Anyway the amount of nitrite in excipients and others will be the key of the risk assessment, especially API or excipient has certain amine moiety.
@GENERAPHARM
Thanks a lot. Even in this example, lactose includes trace amounts of nitrite. Lactose of this brand may be better in quality.
6 Likes
Please excuse my ignorant question but where would the nitrite in the lactose come from? Obviously an impurity, but from which raw material or process step? Thank you for helping me out here.
@BettineBoltres please do not hesitate to place questions in the community. Our community thrive not only on sharing knowledge but also adding clarifying questions.
I’m not familiar myself with the Lactose manufacturing process itself. We do know that Nitrates are naturally found in dairy products.
Maybe @KariA @creekmorejr @JavierFernandez could help us answer this?
Hi, I could help into this. I quote what the following research paper indicates: “…it is possible that these trace impurities could come from processing water, processing steps requiring acid titration, bleaching, and potentially from oxidation of air as the excipient is being heated in a drying process.” Reactive Impurities in Excipients: Profiling, Identification and Mitigation of Drug–Excipient Incompatibility. Under my experience, looking into manufacturing declarations when available the main source is indicated as coming from the oxidation of air in drying procedures (e.g., direct gas fired drying, etc.). Now this is in general, for Lactose I could guess the main sources could come from the water used prior to crystalization and could be too the drying procedure (I have not seen it in direct gas dryers but fluid bed dryers). I hope this helps to your question.
3 Likes
The easiest answer----all plants contain nitrite/nitrate and cows eat grass. In fact one of the concerns about mutagenicity is that Salivary nitrate from dietary or endogenous sources is reduced to nitrite by oral bacteria. In the acidic stomach, nitrite is further reduced to NO and related compounds, which have potential biological activity.
So even humans are a source of nitrite.
1 Like
Ah, ok, I understand now. Sure, I knew about the reduction of nitrate and nitrite in our stomach, but I wasn’t really aware of this. But it makes sense: Lactose is isolated from milk, cows eat grass. Easy as that :-).
Apart from that NO is needed in our body to maintain blood pressure, but of course only in certain amounts.
Thanks
1 Like
I would recommend to talk to you lactose/mcc suppliers directly and you will see that the statement made can be adjusted with factual data
We have talked to two of the biggest MCC manufacturers. One - no response and one unofficial that they had checked one batch for nitrite (present at ppm levels) but did not intend to introduce this as a routine test. Not very promising. We have also found nitrite in excipients where I would have been absolutely certain that there could not be anything.
@GENERAPHARM @Bastiaan @BettineBoltres @Diego @Yosukemino This is a fantastic exchange on a topic that is becoming more and more relevant to finish dosage form producer. Do we know of suppliers that are “doing the best” work when it comes to Excipients?
Maybe we could start a group discussion on best practices and recommendations directly from these producers to get their perspectives. I am happy to coordinate and host the exchange with your collaboration. Any thoughts?
1 Like
Great idea-it would be nice if we could convince excipient manufacturers that there is added value for nitrite free excipients. I know that some companies have introduced special grades of low peroxide materials so there is some precedent for this.
Just to add some color and relevance to what we are all discussing here. If you participated in this morning PhARMA Nitrosamine Workshop (Invite here: PhARMA - Global Workshop on Nitrosamine *FREE*) you heard Dr. Aisar Atrakchi from FDA (CDER) presenting:
-
Case study #1: Nitrites in the formulation reacted with an API to form a nitrosamine drug substance-related impurity. The review team (FDA) concluded that the impurity of interest had sufficient data to indicate a mutagenic risk, BUT inadequate data on carcinogenic potential. The review team recommended that the acceptable intake (AI) should not be adjusted using ‘Less-than-lifetime’ principles.
-
Data Gps: Current and Future Research Needs: Assess formulation issues and how specific excipients may contribute to the formation of NAs in a drug product
2 Likes
Sure, I also think it is a great idea, but belonging to the primary packaging suppliers, I cannot speak for the excipient suppliers. If we have any excipient suppliers in this group, I hope they would be available for this exchange.
I completely agree with you about the difficulties of removing nitrites from excipients and I can assure you that as finished dosage experts we are looking into a number of ways of reducing risk, including nitrite scavenging and process parameters. However, we have recently found quite surprising levels of nitrite in some excipients - in excess of 4000 ppb.
What would be very helpful from excipient manufacturers would be to have some hard data. Is it too much to ask for nitrite content to be added to excipient specifications? After all you are already doing such things as elemental impurities.
So far we have found it almost impossible to obtain any nitrite/nitrate data from excipient manufacturers.
From my side too I can say that excipients that makes no sense to have nitrites have come possitive. Quite interesting actually.
I also have seen quite difficult to obtain information on nitrites from excipients and adding into the specification without knowing where the nitrite source comes from would result in a lot of OOS (what limit should be set then? Lot of questions there). Nevertheless, we have received that information in some cases.
Some manufacturers have hundreds of excipients into their portfolio, even if the cost of testing nitrite I have seen seems ok, for hundreds it could be a problem. However, that is where it could be of use from the excipients manufacturers to evaluate what excipients makes sense to be tested and have some raw data into it. Even if the responsability is into the MAH, how can we set up also how much nitrite scavengers, etc if at first we dont know have much nitrite we expect, etc etc.
There is quite a lot of things to analize that should be said as now as part of finding a common medium point between every stakeholder.
I am glad your company is addressing the issue. Unfortunately we are getting responses such as “we do not deliberately add nitrosamines to our product” from certain suppliers.
This issue will separate the good suppliers from the bad and is a real business opportunity in my opinion.
The main problem at this time is really the lack of available knowledge regarding nitrite content and without that we cannot really address their real risk contribution to nitrosamine content in finished dosage forms.