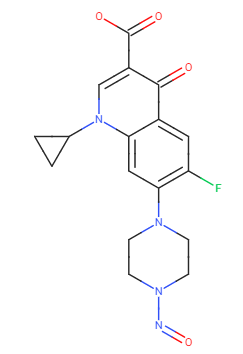
Anyone having idea about AI limit of Nitroso Ciprofloxacin?
What could be the AI limit as per read across approach?
Hello:
I have the same question ![]() . I hope someone knowledgeable can help us. Assume a limit of 18 or 1300 (for similarity with the nitroso piperazine), It is a big difference.
. I hope someone knowledgeable can help us. Assume a limit of 18 or 1300 (for similarity with the nitroso piperazine), It is a big difference.
@IreneNS
Of course it’s a big difference. However in many cases the default limit of 18 ng is irrealistic and not justified by a read-across approach.
I hope that this article (often cited in this forum) may be useful:
In your case, the nitroso-Ciprofloxacin belongs to Group 10, with a proposed “group AI” of 153 ng/day.
I think (and hope!) that such limit should be accepted by most of Health Authorities
thank you!!! We will try to be admitted to a higher limit. If we have a response, I will put what the authorities tell us in case it is useful.
It seems that regulatory bodies are inquiring manufacturers to demonstrate the absence of Nitroso-Ciprofloxacin.
SMILES: O=NN1CCN(C2=C(F)C=C(C(C(C(O)=O)=CN3C4CC4)=O)C3=C2)CC1
Just for context, I found an article from water research that study the reactivity of Ciprofloxacin under nitrosating conditions and demonstrated the formation of the Nitroso-compound.
Perhaps the recent post on the FDA structural similarity poster can provide guidance. The authors also refer to the Dobo et al. publication
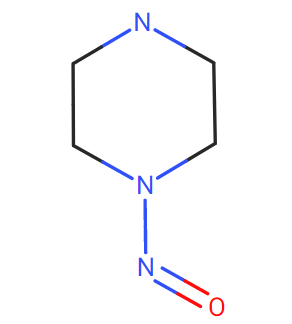
1-Nitrosopiperazine can be a surrogate.
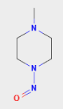
1-methyl-4-nitrosopiperazine can also be consider. Moreover EMA and Health Canada has published limit for 1-methyl-4-nitrosopiperazine.
Health Canada limit: 96.0 ng/day while EMA limit: 26.5 ng/day
I would like report a new article on this topic:
Setting limits for N-nitrosamines in drugs: A defined approach based on read-across and structure-activity relationship for N-nitrosopiperazine impurities
Thanks you a lot! It´s a tricky questions…
We may not use AI 1300 ng/day, because N-nitroso ciprfloxacin has pipeazine ring not piperidine ring
AI- in still evaluation
May i ask the source of AI 140ng/day? read-across from which?
Please note that is based on my subjective opinion (It can be subjected to correction)- Not published value. Still in the process of evaluation. Conservatively that is the least value that can be defended considering all piperazine based nitrosamines.
Read across from TriMNP will give an AI of 153 ng/day. TriMNP is the most potent nitroso-piperazine in the LCDB and it has robust carcinogenicity data, so it should be accepted as a valid surrogate for other nitroso-piperazines such as nitroso-ciprofloxacin.
Thanks @conudel. Agree to some extent. It looks like the most potent among all N-nitroso piperazines is 1-methyl-4-nitrosopiperazine. There is difference between 1,2,6-trimethyl-4-nitrosopiperazine and 1-methyl-4-nitrosopiperazine in terms of carcinogenicity.
Structurally 1-methyl-4-nitrosopiperazine looks more closer to N-nitroso Ciprofloxacin.
with absence of beta methyl substituents.
The MW difference should also be accounted for when doing a read across. You’re expecting the functional groups to contribute to the toxicity, not the MW generically.
Agree. Molecular weight has role.
Summary
This text will be hidden
Agree MW also important. The problem with NDSRIs are it is tough to get both MW wise and structural wise the closer analogues.
Let me explain better. Don’t worry about the MW when identifying the closest surrogate. Sure, steric hinderances, etc. should be accounted for, but the last step in the process is:
AI (surrogate) * MW (molecule of interest) / MW (surrogate)
Dear @jason.brown. I Understand what you are referring to. Definitely one should not worry about MW for finding analogue because for NDSRI it is tough.
In regard to Molecular weight correction with respect to AI- could you please let me know does agency accepting such approaches.