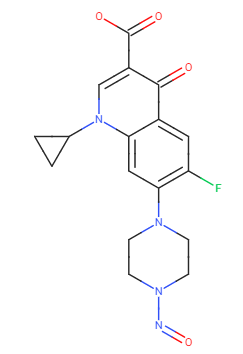
It seems that regulatory bodies are inquiring manufacturers to demonstrate the absence of Nitroso-Ciprofloxacin.
SMILES: O=NN1CCN(C2=C(F)C=C(C(C(C(O)=O)=CN3C4CC4)=O)C3=C2)CC1
Just for context, I found an article from water research that study the reactivity of Ciprofloxacin under nitrosating conditions and demonstrated the formation of the Nitroso-compound.
Perhaps the recent post on the FDA structural similarity poster can provide guidance. The authors also refer to the Dobo et al. publication