Recently we received Metformin API from two different supplier and in one supplier NDMA is getting about the 0.034 ppm which is nearing the limit of 0.038 ppm(if we consider the MDD of 2550).
As supplier is not agreeing to us can we increase the limit and what could be the probable justifications.
Dear TAUSEEF SIDDIQI,
just change the supplier ![]()
It is not logical for the API supplier to set the specification limit for the API near the limit.
In similar cases we have demand to set the API specification around 10% of the nominal spec. limit and in most cases this have been accepted, even as an ‘‘internal limit’’.
If your supplier is denying to change the spec. limit, you could demand from him to set an internal limit for the batches which are going to be sent to you e.g. 0.003ppm.
It is not possible to change the limit of NDMA, it is one of the most studied nitrosamines and its limit is well established, at least up to now.
Please, also note that the quantity of DMA (dimethylamine, as starting material) is also critical in the API of Metformin.
best regards
Christos
Hi chris
Many thanks for your reply.
Surely supplier will be changed but to defend himself he shared the analysis with results about 0.003 ppm. When i investigated i found he have only used quantifier whereas there there was no qualifier to confirm the same ndma.
My question is as i have seen different monographs and available methods by authorities they have used both quantifier as well as qualifier. Is it mandatory to apply both or only one is workable??
I believe @chrischar has made a very important point. Remember that the limits of impurities are intended for the finished product. Regulators will evaluate the finished products against those accepted standards.
The risk assessment and robust evaluation of the ‘finished product’ will indicate if the specifications for your suppliers need to be adjusted. You might need to reconsider new suppliers. Please do not focus solely on the nitrosamines in the active ingredient (API), remember the case of DMA in Metformin. One needs to consider the nitrosamines AND the precursors that could form further nitrosamines downstream in the process.
The solution is not to find a justification for a higher limit because the supplier doesn’t want to comply with a tighter internal limit.
dear tsiddiqi
I totally agree with both Christos and Naiffer.
Based on previous experience, I would like to add a note from my side. Please always keep in mind that as Naiffer said, Regulators will evaluate the finished products against those accepted standards. But please note that this is related to your product during stability as well. and perhaps your NDMA go a bit higher during storage. So, such marginal levels in API would definitely be risky for your FP.
What’s more, there are plenty of Metformin suppliers with well controlled NDMA levels in API as to mitigate this risk, but please don’t skip a quick control of your API material from your end too.
kind regards,
Eleni.
Dear tsiddiqi,
as far as i now, qualifier is not mandatory to be presented. But the usage of both quantifier and qualifier secure the quality of the presented method.
Neverthelss, even if the API supplier presents a very good trend of low values of NDMA you cannot be safe if the limit is so high.
For this reason, they should at least agree with you in an internal limit around the 10% of the spec. limit.
best regards
Christos
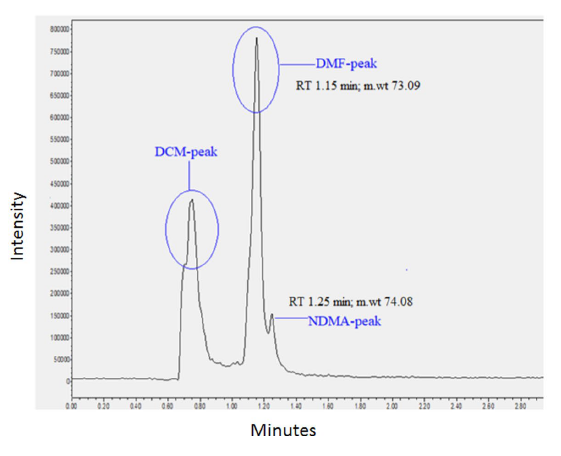
May I invite you to take a closer look at some data?
Take a look at this chromatogram I’m sharing:
It was generated as part of an in-use stability study involving metformin products combined with glibenclamide, glipizide, pioglitazone, alogliptin, and linagliptin. The samples were stored for 3 months at 30 °C / 75% RH and, at the end of this period, were analyzed for the presence of NDMA.
Detection was performed by LC-MS, using ESI in positive mode, with a single quadrupole monitoring the precursor ion at m/z 75.06 (NDMA).
Now I ask you: would you feel confident making a regulatory decision based on this data?
Let me walk you through my reasoning, from someone who’s also routinely evaluating this type of data.
The literature itself — including the article from which this chromatogram originated — shows that DMF can interfere with NDMA detection. Even at much lower concentrations, DMF can produce a signal that suppresses NDMA, masking the real risk. The result may appear to be below the limit, but still be compromised.
And this brings us to the core of your question: is using only a single quantification channel sufficient?
In the article in question, only the precursor ion is monitored. When we’re dealing with potentially carcinogenic impurities and extremely tight limits, relying solely on m/z 75.06 — on a unit-resolution system like a single quadrupole — is risky. Especially since this m/z can be shared with other substances present in the sample.
Another critical point: nitrosamines like NDMA tend to fragment at the ion source, losing NO (30 Da), which compromises sensitivity and increases noise. This makes the scenario even more challenging on instruments with intrinsic limitations in selectivity (single quad).
With that in mind, working with quantification and qualification channels means adding an extra layer of selectivity and confidence. What I bring to this discussion is the understanding that using two channels is not a regulatory requirement or a checklist item — it is a safeguard against false negatives and hidden interferences.
When we’re dealing with health risks, what matters most is trusting the data. And trusting the data doesn’t just mean accepting the final number — it means understanding how that number was produced and whether it holds up under audit or in a critical decision-making scenario.
Lucas Maciel
The article is available here: Patient In-Use Stability Testing of FDA-Approved Metformin Combination Products for N-Nitrosamine Impurity
Dear Lucas
Many thanks for the positive reply and i second you on believing and recommending using as qualifier as well as quantifier both to have precise data.
Can i add one more question to the ongoing query that how come supplier had ion source as EI for GCMS and the precursor ion is 74.
It should be 73 or 75 correct??
Dear Tauseefy,
In this case, the m/z 74 reported by the supplier is correct. The equipment uses an Electron Ionization (EI) source, commonly employed in GC-MS systems.
I won’t go into the ionization mechanism in detail here, but essentially, EI involves ionization of atoms or molecules by electrons, which are typically accelerated in order to remove one or more electrons from the target species.
Because the mass of the electron is negligible compared to the molecular mass, the resulting ion’s m/z closely reflects the actual molecular weight. That’s why we refer to the molecular ion (not protonated molecule) in this context — and in the case of NDMA, this corresponds to m/z 74.
excellent comment, muito obrigado Lukas!!
I strongly endorse the comments received. It is always advisable to control impurities to below the limit of detection (LOD) or ensure they are not detected, particularly in the active pharmaceutical ingredient (API), as drug product excipients may contain traces of nitrites/nitrates—which may not be practically possible to eliminate completely—and the likelihood of impurity formation during the shelf life is higher.
Hi all
I found one surprising observation that for the same API when we tested Nitrosoamines with different method NDMA was not detected.But when we are testing single nitrosoamines then it with found to be at the MDD limit.
What could be the possible reasons behind it,does detecting of nitrosoamines varies from column,instruments and methods etc.
dear tsiddiqi
I am not sure if this helps you but please note that there are established method in USP for NDMA determination in Metformin Drug Substance and Drug Product.
Dear Colleagues,
What I understand during handling of Metformin that there is not NDMA present in Metformin, NDMA impurity generates in formulation when DMA present in Metformin and nitrates present in excipient reacts. So essentially, we need to control the DMA content in Metformin less than Pharmacopeial limit of 500 ppm. Most of the analysis of Pure Metformin API will show NDMA content as not detected.
Hope this helps, I will be happy to get comments on this discussion.
Regards,
I believe you will have to closely look at your method and find out the source for this inconsistency.
Lucas pointed out that DMF can interfere with NDMA analysis on LCMS. This is applicable for GCMS also. Note that DMF has a limit of 880 ppm which is significantly higher than NDMA and even trace amounts of DMF can interfere with your detection.
I suggest you can include DMF at 880 ppm in your specificity experiment to ensure that there is no interference. Perhaps even a spiking experiment with DMF may help you.
Dear Shamkant,
i totally agree with you, in most cases the API manufacturers have done very nice job on the risk mitigation of NDMA resulting in ‘‘not detected’’ results.
On the other hand, the API manufacturers usually following the pharmacopeia limit for DMA, 500ppm (as you said) which is very high for nitrosamine cases.
My advice on this is to discuss with the API manufacturer in order to provide you results of DMA content, using a method with an LoQ less than 100 ppm at least.
kind regards
Christos
Dear elenipoliti
In general chapter 1469 i cannot find method for metformin. Can you kindly share the monograph which you are talking about.
dear tsiddiqi
please check Table 5 CDER Nitrosamine Impurity Acceptable Intake Limits | FDA
best regards,
Eleni
Dear Tauseefy,
In this case, I’ll need more details about the methodology so that I can perform a proper risk assessment. From there, I’ll be able to better understand what might be happening and guide you accordingly.
BR,
Lucas Maciel
In addition to this, there are some methods published by the EDQM that use GCMS and GCMS/MS for NDMA determination in metformin. They can also be useful.