Dr. Dave Elder wrote an article this week in European Pharmaceutical Review - “Less than lifetime limits for N -nitrosamine mutagenic impurities” Here, Dave Elder discusses the determination of less than lifetime (LTL) limits for highly potent N -nitrosamine compounds and how to ensure safety in dosing.
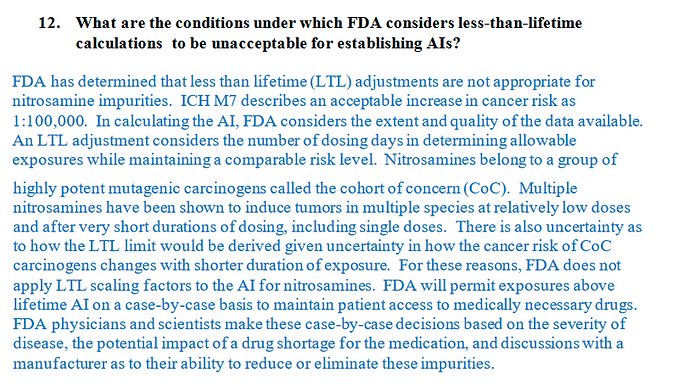
Thank you Naiffer for sharing this interesting review, have anyone applied the LTL for large drug volumes and got it accepted by FDA or MoH in EU? Since the guidance is pretty strict on applying life time limits I was wondering if there is any precedence for authorities to accept the LTL application?
thank you
EU agencies has not accepted the LTL limits so far in our experience. However, I could see the SAHPRA has provided some LTL limits in their draft guidance on Nitrosamines. “Communication to Industry on Nitrosamine Review for New Applications and Registered Products including Biologicals – Document for comment”
Unfortunately, the draft guidance is no more available on the SAHPRA website. It is not clear how SAHPRA has arrived such LTL limits.
If anyone can share the guidance if previously saved for further discussion. I will see if I could get hold of it.
Thanks,
Sumit
What should be strategy for drug like Ketamine which is indicated as the sole anesthetic agent for diagnostic and surgical procedures is best suited for short procedures (mostly single dose). For this can less than life time approach justified.
Can FDA response to such query through control correspondence.
Also even if we are controlling nitrosamine impurity per drug product but in practice patient may be taking multiple medication. In that case determination of risk is difficult
Thanks
Hi, @sameer. I think the following is helpful. The original document is here. The case by case based approach is acceptable.
thank you it is really helpful
Nitrosamine-SAHPRA.pdf (1.1 MB)
@Naiffer_Host @Rawaa @sameer @Yosukemino
Dear Experts,
SAHPRA, South Africa has released the final guidance on NITROSAMINE COMMUNICATION (enclosed) and is largely in line with the EMA and FDA guidances. Nitrosamines limits are adopted from EMA Q&A documents.
Some additional flexibilities are listed below:
-
Applicants can submit variations to their API and/or FPP (e.g. manufacturing process, controls and specifications, product formulation, raw materials and packaging) during the pre-registration phase if nitrosamines are detected above acceptable intake limits.
-
Acceptability of less than life time approach on case by case basis with SAHPRA consultation and well defined treatment duration adjusted AI limits.
-
SAHPRA also accepts limits approved by authorities which it aligns itself with.
Happy reading!
Thanks & regards,
Sumit
A great summary and thank-you