You do not want to miss this webinar with the participation of @schlinjo1975 and @Ulrich on Sep 28th
Description:
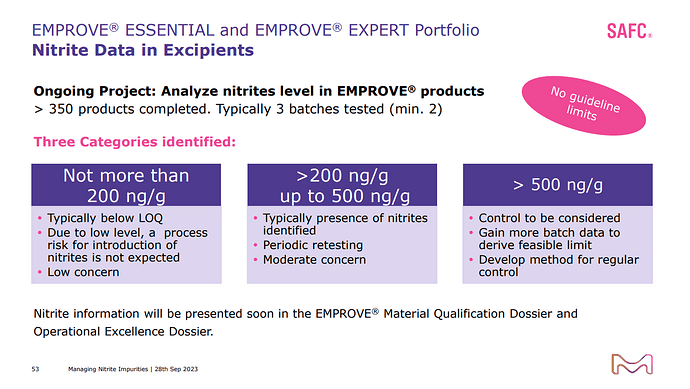
The presence of nitrites in excipients evolved as a hot topic among regulatory agencies across the world. This Nitrites in excipients are seen as a risk factor to form nitrosamines in the presence of vulnerable amines during drug product manufacturing process and storage. Health Authorities worldwide imposed recalls to drug products. Our data indicate that the presence of nitrosamines in pharmaceuticals is likely more prevalent than originally expected. This webinar will review the current regulatory considerations, the role of nitrites, the analytical controls. The view of a drug product manufaturer on the related risk mitigation strategy and a case study on risk mitigation is presented.
Free Registration: Managing Nitrite Impurities: A Combined Supplier-Manufacturer View to Nitrosamine Risks