Dear Sir,
What will be the Maximum Daily dose for Dorzolamide Hydrochloride to be considered for Drug Substance and for Finished Formulation for NDSRi limit calculation for N-Nitroso Dorzolamide.
As per the available SPL, Maximum daily dose of Dorzolamide Eye Drops (20 mg/ml): 1 drop in the affected eye(s) 3 times daily i.e. total 3 drops in the affected eye(s).
Considering 1 drop is 0.1ml then 0.3ml total daily dose in each affected eye. So 0.6mg is maximum daily dose in the each affected eye.
if we consider application to both eye, it’s coming 1.2 mg.
Note: Each ml contains 22.26 mg of dorzolamide hydrochloride equivalent to 20 mg dorzolamide.
Pls suggest.
Thanks
Shailesh V
1 Like
I believe this is a multi dose container.
20 mg/mL is the drug concentration, It is not your MDD.
1 mL of solution contains 20 mg of active, hence, 0.6 mL of solution corresponds to 12 mg of drug, not 1.2 mg.
Thank you very much for your reply
Dear Shailesh,
Following points to be considered:
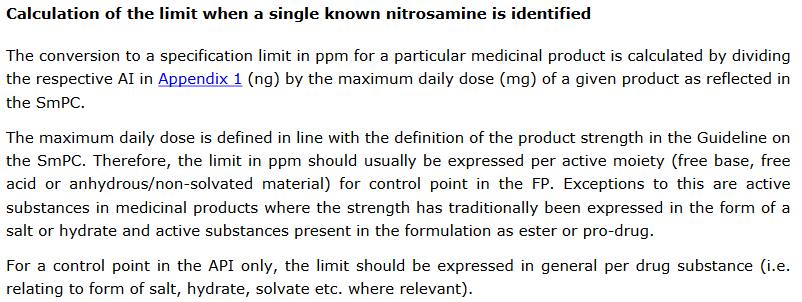
A) According to EMA/409815/2020 Rev.19 you have to take the SmPC stated strength of the API into consideration (“per active moiety”):
B) You then take the respective AI value as published in the Appendix 1; in our case N-nitroso-dorzolamide = 100 ng/day.
C) Now you need MDD.
Either you take the “standard MDD” via the published WHO ATC-Code. This should be I think for ATC S01EC03 = 0.3 ml.
Or
In case you have the supporting data, your specific MDD can given as measured based upon drop sizes. In this case often the MDD is lower as drop sizes tend to be in the lower range.
Dependent on how you argue (“safety margin, ophthalmic dosage form with limited uptake by the conjunctival sac, nasolacrimal occlusion”) you may present your case individually.
Hope this helps…
1 Like
Thank you very much for your reply