Interesting recent open access paper on the usage of stainless steel milling balls (10 mm diameter) for lab use of nitrosamine synthesis in what the authors describe as a mechanochemical reaction:
https://doi.org/10.1002/cssc.202301034
The conversions are high and reactions not reported as unselective, whether molecules with C-nitration risks are included (cf. risk to yield nitro-APIs or nitronitroso-APIs as by-products when executing the classic literature processes in solution to obtain reference standards, occasionally too little to derisk the NDSRI but too much to have an efficient reference synthesis).
Some NDSRIs or structures close to NDSRIs are included in the examples.
If repeatable and scaleable, could this be a way towards more efficient synthesis of stable isotope labeled nitrosamines by treating the precursors with 15N-NaNO2 without losing expensive labeled reference material to undesired side reactions?
In the supporting information the authors discuss an example where the conversion in solution was only 21%
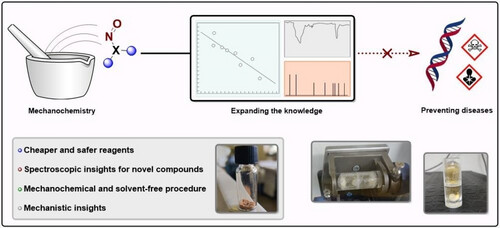
Procedure for nitrosamine synthesis:
A 10 mL stainless steel jar equipped with one stainless steel milling ball (10 mm diameter) was filled with amine (2.00 mmol), NaNO2 (3.00 mmol or 4.00 mmol) and NaHSO4 ⋅ H2O (2.61 mmol or 3.48 mmol). The vessel was then closed and the mechanochemical reaction was conducted for 60 or 120 min at 30 Hz. At the end of the reaction, the crude was recovered as a solid in a beaker, dissolved in ethyl acetate or in ethanol (8 mL), and filtered on paper. Lastly, the solvent was removed under reduced pressure to afford the pure nitrosamines .
The suitability of these type of experiments to understand observed formation in solid dose medicines probably needs to be explored further, including finetuning of the amount of reagents used, or a possibility to with extrapolation subject solid powder formulations to the mechanical process. For stress experiments it can be interesting to speed up nitrosation with metal ball effects, but does it generate fully extrapolatable selectivity and nitrosating species relativity compared to non-mechanical solid phase formation of NDSRIs?
If the metal ball usage is sometimes better for investigative purposes than some of the existing processes remains unclear, as the authors only compared stoichiometric processes in solution and solid phase.
Cf. Yamamoto 2023, Ashworth 2023, Nanda 2023 for studying nitrosation in solution.
Cf. Nanda 2023, Golob 2023, Moser 2023, Teasdale 2022 for studies on R&D/pilot tablets
Cf. Cioc 2023 evaluating such approaches
Is the stainless steel ball use the answer to faster but extrapolatable small scale experimentation? Or is this only the case when a match with the mechanical solid-phase root cause is seen?
Has anyone tried this?
Of note, the authors also explored possible mechanistic differences between nitrosation in solution compared to the solid phase (which is also not fully waterfree). What is the nitrosating species from HNO2 is always a discussion point, NO+ would be most important for the solid phase chemistry, but N2O3 relevance cannot be fully excluded. The grinding effect from the metal balls helps to keep a high decomposition rate of HNO2 yielding NO+.
supporting information
Higher conversions in the solid state experiments compared to in solution chemistry in some case show that solution models sustain too much HNO3, slowing down the access to the nitrosating agents compared to the metal ball effect in the solid phase.
The mechanochemical process mostly works for less basic nitrosamines as these have lower competitive acid-base activity with HNO2.