1 mM nitrite is high, I don’t know if we have to use 96 ng/day here and if the model shouldn’t be finetuned to intake together with a meal (not just keeping cured meat-like nitrite exposure, but also antioxidants and amine scavengers).
It disturbs me a little bit that the point of departure is 96 ng NDMA/day as acceptable exposure without nuancing. The RAIL for exogenous contamination in medicines is not per se the basis for evaluation of endogenous formation. When we start to go endogenously I think this is moving away from unintegrated exposure scenarios and looking more holistically, with reduced possibility to distinct on the origin of NDMA and also looking at combinations of factors (e.g. nitrite from food) and faith, leading to more advanced exposure models for balancing risks.
The nitrite in the stomach is obviously not going to come from the medicine and nitrite preservative use is maybe a little bit too widespread to add this as meal restriction on the leaflet, so assessment of the risks merges the food and medicine perspective. If you want to look at this from a regulatory toxicology perspective (which is done by citing only pharma-regulation linked AI for NDMA), food and pharma have to agree first on the acceptable exposure to NDMA? This is not the case today, see for example:
In pharma we are often used to looking at exposure unintegrated, also for multi- (endo, exo or end/exo) root cause impurities (typically ignoring biokinetics and other sources of risks), but typically pay the price for this limitation with conservative limits/extra precaution. As endogenous nitrosamine formation is not new in literature, also not linked to API, possibly the conservative RAIL for medicines (for NDMA, CPCA,…) can be interpreted in this precautionary sense. Increasing quantification of endogenous risk to in the future allow to couple back to exogenous limits and thus more holistic models is thus useful.
It would have been useful not only to compare with the exogenous NDMA limits for pharma, but also with NDMA endogenous formation baseline levels and NDMA exposure from foods which can be in totality much higher than 96 ng/day.
I found it strange that the fact that metformin has to be taken with meals is seen per definition as increased risk (or as subscribing the relevance of the study conditions used), as this would require establishing that the average meal is more contributing to nitrite risks than to antioxidant-linked or alternative amine-scavenger derisking (e.g. N-terminal proline) for the scenario? Especially because the tests are done in vitro with simulated gastric fluid without varying parameters like spiking with proline or ascorbic acid (see for example), but while using high nitrite concentrations (simulating only the worst case elements of a meal). Even the exposure to nitrites as preservatives in cured meat goes hand in hand with the dosing of N-terminal proline or other biogenic amines, whereas also this type of preservative use has become increasingly regulated (quite recently updated in Europe in fact). Nitrosamines risks for high nitrite high amine foods is a study field on its own.
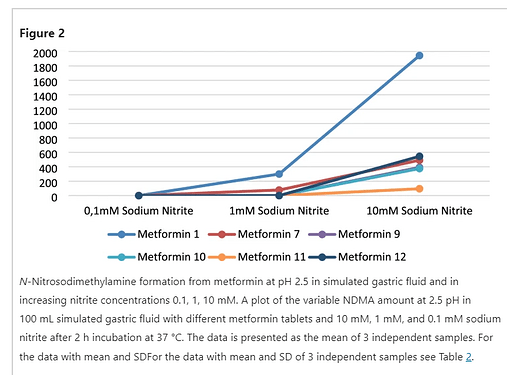
For stomach nitrites, the “closest concentration to physiologic conditions used, 1 mM” seems quite high (or (cured meat) meal-like)?
0.100 L volume gastric fluid (fasting…) x 1/1000 M nitrites (non-fasting…) = 0.0001 mol x 46 g/mol = 4.6 mg <=> The ADI of nitrites is 0.07 mg/kg bw
/ 150 mg nitrites/kg food (max. nitrite preservative use in Europe) = 31 g of high nitrite food product
On stomach nitrite concentration see also review in link. 1 mM is really a high nitrite concentration. So considering max. 400 ng NDMA/tablet for the tested conditions (I guess 1-2 tablets/day) (and the discussions on applicable acceptable exposure for NDMA and real exposure) with a possibility to optimize further for the meal scenario, the data might as well contribute to derisking in the end. So fully support more work is indeed required.
What do we think was the rationale behind selecting pH 2.5? (Empty stomach is pH 1.5, pH 3.15 usually selected for maximum conversion; selected something in the middle between range of pH 1.5-3.5 for stomach pH)?
Would have been nice to understand the variability in endogenous formation link with formula composition (now only distributors and lot numbers are provided in SI, no checks on antioxidant presence, API concentration, solubility, possible interaction with the simulation fluid etc.). Sun Pharmaceuticals tablets are most resistant in this test.
Overall I found the EMA-funded study designs maybe more on point or transparent, papers are apparently in the submission phase. Looking forward to the results to combine insights, anticipating a new chapter in RAIL discussions if the endogenous field gets further increased attention?