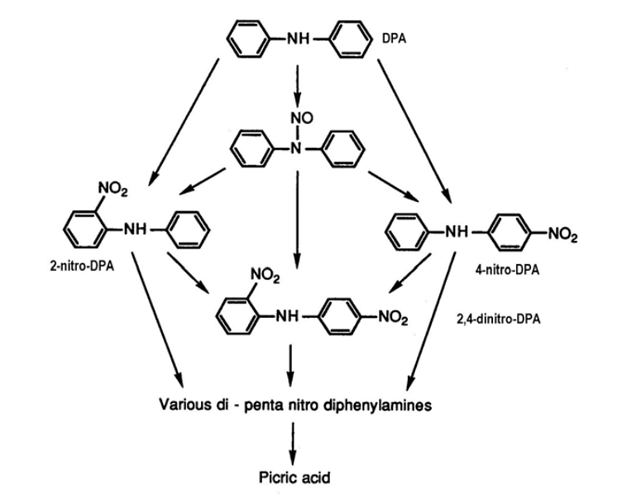
Just wondering if anybody in the community has read or used microcalorimetry to explore Nitrosamine Formation Chemistry.
Microcalorimetry is a growing technique complementary to DSC for the characterisation of pharmaceuticals. A larger sample volume and high sensitivity mean that phenomena of very low energy (unmeasurable by DSC) may be studied. The instrument’s output is measured by the rate of heat change (dq/dt) as a function of time with a high sensitivity better than 0.1 μW. Microcalorimetry has been used to isolated systems in specific atmospheres; or for batch mode where reactants are mixed in the calorimeter. The method is used for polymorphic interpretation and for quantitation. Microcalorimetry is useful for stability and compatibility predictions.