In EMA guidelines list nitrosamines linked to molecular structures with a morpholine ring and also a nitroso-morpholine. In “complex” molecular structures nitrosation does not always occur at the morpholine ring. What do you think of this structure? Is nitrosation possible?
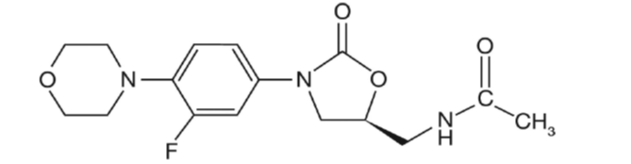
N-nitrosation on the morpholine ring in this case may not be possible. There are no other vulnerable amines, so N-nitrosation doesn’t seem to be possible anywhere else on this molecule. Formation of the N-nitrosamide (nitrosamides are out of the scope of CPCA) is possible in non-aqueous media or in presence of powerful nitrosating agents.
In this case I would definitely check whether there is a potential carry over of morpholine in the API from the synthesis. That may be the risk there
You should also check for potential impurities, in molecules with nitrogen-containing heterocycles, open-ring impurities may contain secondary amines.
I would however mention the risk of dealkylative nitrosation of the tertiary amine, with the possible generation of nitroso-morpholine, and a nitroso-aldehyde derivative.
Further, the stability of carbamate moiety should be assessed and commented as by hydrolysis would generate a secondary amine, which could be an impurity of the API and a degradant in the drug product.
Perhaps something to add, that morphline ring came somewhere in the synthesis of the API. Would be interesting to understand what are the actual values in the final API, as perhaps in the finished product they could react and form the corresponding nitrosamine.
Considering that ICH Q3A/B levels are in %, and for nitrosamines from personal experience double digits ppm or less of a secondary alkyl amine are meaninful for a Category 1 and 2 CPCA compound. (Here 127 ng/day as per read-across).
What do you mean with “nitroso-aldehyde derivative”?
A compound theoretically resulting by cleavage of the morpholine cycle, including aldehyde and nitrosamine groups, as
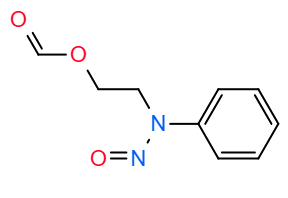
Thank a lot for all suggestion