Abstract
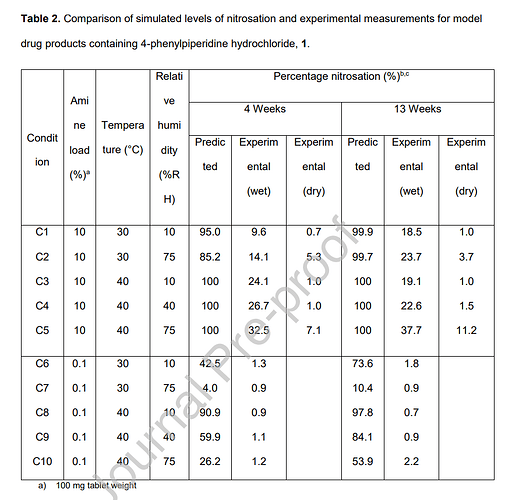
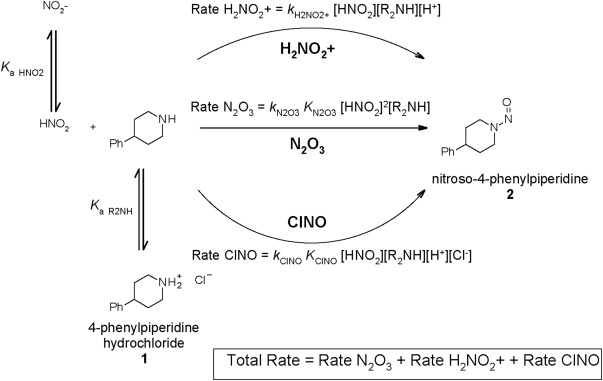
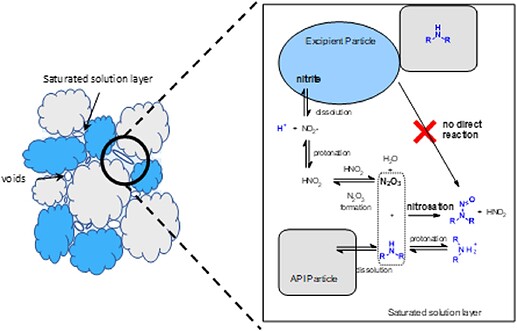
Following the discovery of *N-*nitrosamines in a range of medicinal products, regulatory agencies have required marketing authorization holders to undertake risk assessments for the presence of N-nitrosamines in their drug products. This work focusses on a solution phase kinetic model of secondary amine nitrosation that may be applied through the concept of a saturated solution layer to consider the formation of N-nitrosamines in a solid drug product. The conservative assumptions made in defining the reactant concentrations bias the model to overpredict the level of N-nitrosamine formed. This overprediction is demonstrated when model predictions are compared to testing data for the formation of nitroso-4-phenyl piperidine in a model drug product. Additionally, comparison of the model predictions to product testing data for two Nitrosamine Drug Substance Related Impurities (NDSRIs), a nitrosated β-blocker and the nitrosamine of a dialkylamine related substance impurity, further confirm the tendency of the model to overpredict. These results demonstrate that the conservative model has utility in the context of a drug product nitrosamine risk assessment. Extension of the model to consider competing nitrosation reactions occurring within a drug product is discussed alongside the impact of reactant availability on the predicted rate of nitrosation.