Can heterocyclic aromatic ring form N-nitrosamine impurity?
Yes, under the right conditions it can be formed.
@ghanshyam.joshi Do you have any specific example that you would like to share?
Do you mean a pyrrole-type nitrogen, c1cccn1N=O ? These can definitely nitrosate but are not expected to undergo the a-hydroxylation mechanism that leads to concern, since cleavage of the aromatic ring to form the diazonium is very unlikely.
There is an alternative mechanism of mutagenicity/carcinogenicity, reduction via nitroreductases to form the hydroxylamine and thence a nitrenium ion - and comparable adducts to aromatic amines - but I would not expect them to be CoC.
Nitrosated heteroaromatic amines, e.g. c1nccnc1N(C)N=O, are expected to behave similarly to nitrosomethylaniline, and thus need to be treated as CoC.
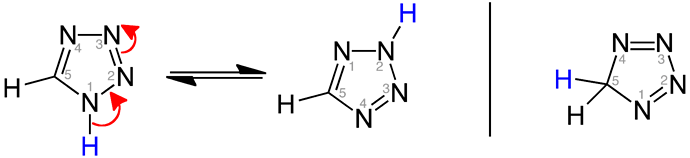
Here you can find some examples of Nitrosamine drug substance-related impurities (NDSRIs) or other impurities where the tetrazole ring contains a N-nitroso group:
Valsartan Tetrazole nitrosamine impurity
Also in this case, there is any alfa-hydrogen, so they are not expected to undergo the alfa-hydroxylation mechanism. However, we should consider that the tautomerism of tetrazoles may complicate the framework.
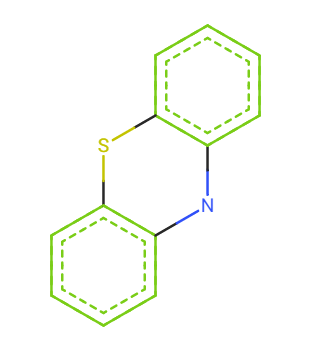
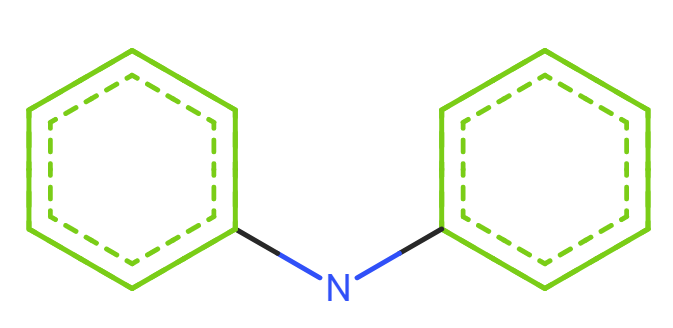
Phenothiazine and diphenylamine
Phenothiazine
SMILES: c1ccc2c(c1)Nc3ccccc3S2
Diphenylamine
SMILES: c1ccc(cc1)Nc2ccccc2
Nitrosoirbesartan is free from nitrosation in ordinary.
Yes.
Eg. N Nitroso Zolmitriptan
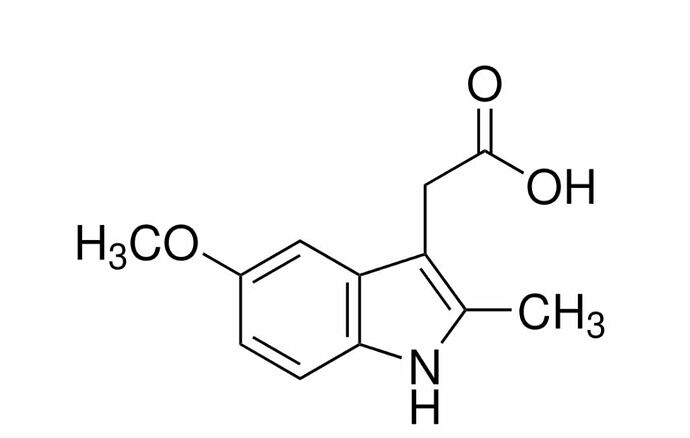
Can Indole related compounds form N-Nitrosamine?
Eg: 5-Methoxy-2-methyl-3-indole acetic acid.
As per the FDA guidance, the predicted carcinogenic potency categorization approach does not apply to NDSRIs where the N-nitroso group is within an aromatic ring. What guidance or limit to be followed for these N-Nitrosamines?
Hello everyone,
I am also dealing with this issue at the moment.
In my special case it concerns metronidazole, used in tablets and in suppositories and gel.
Can anyone give me an answer?
For both of these compounds, even where they can hydroxylate adjacent to the nitrogen, no diazonium can be formed (because the destruction of the aromatic system would result). As a result, while they can still be mutagenic (bacterial nitro-reductases form a hydroxylamine, whence they share a pathway and Ames profile with the aromatic nitro and amine compounds (though nitroso and nitro don’t need S9, the aromatic amines do)), they are not likely to be of potency worthy of the cohort of concern. This moves them to conventional ICH M7 control.
This is discussed in more detail in my recent paper here: https://pubs.acs.org/doi/full/10.1021/acs.oprd.3c00008
Thanks a lot @David. It is very helpful.
Dear Sir,
The CPCA guideline mentions the following:
"Additionally, the potency categorization approach does not apply to N-nitrosamines where the N-nitroso group is attached to a nitrogen within a hetero aromatic ring (e.g., nitrosated indole)."
How is the AI of the above Nitrosamines calculated? Do we take the “worst case” 18ng/day?
Thank you in advance.
Has this question that you posed, which is a good question in my view, ever been answered???
Please read the previous discussion…
This means a TTC of 1500 ng/day.
Kind regards
Waiting answer with good examples
what about pyridine - is it easily formed the connection of the nitroso moiety with nitrogen of pyridine? I have only found a few examples in very harsh conditions where N-nitrosopyridinium formed.