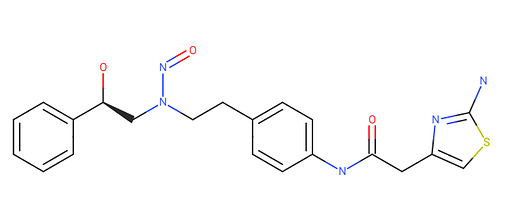
any idea about AI/TD50 of N-Nitroso Mirabegron.
Can we use read across approach to derive its AI for regulatory submission ??
SMILES: O=C(Nc1ccc(cc1)CCNC[C@H](O)c2ccccc2)Cc3nc(sc3)N
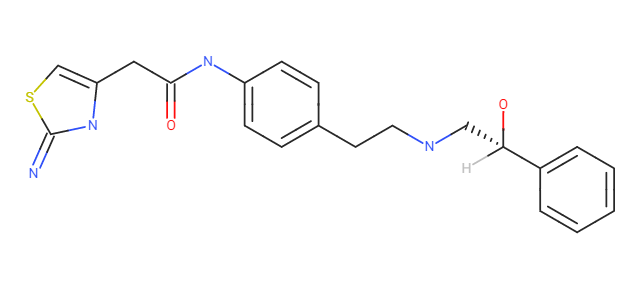
SMILES: O=C(CC1=CSC(N)=N1)NC(C=C2)=CC=C2CCN(N=O)C[C@H](O)C3=CC=CC=C3
Hello everyone.
I am currently working on NDSRIs, including N-nitroso Mirabegron. This impurity is already reported in literature where the NAP test is used for impurity generation. Trying the same, I was unable to generate the impurity. I think in this case, maybe the type of solvent used (depending on drug solubility) to prepare the sample solution reflects an impact on impurity formation. Is it so?
What can be the possible reasons?
I need your valuable insights for the same.
The Mirabegron drug product turns gel like when water is added,how to determine NDSRIs in this
You can use water:Methanol(50:50) for 1mg/ml concentartion , sample will be dissolved easily
This post is relevant now that Japan has reported a market withdrawal due to the presence of N-nitroso Mirabegron
Keep in mind that if a higher concentration is needed, but not achievable, due to solubility, SPE can be used to that end in addition to cleaning up the excipients from the prep