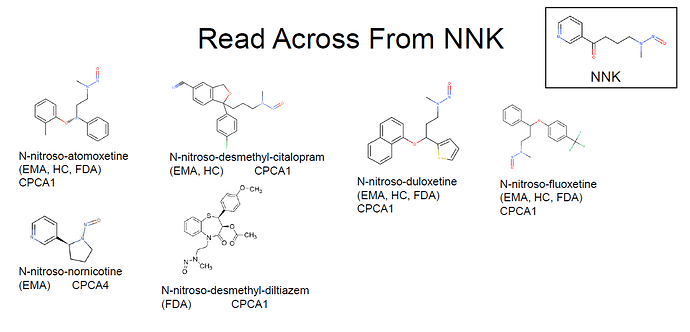
FDA recently added nitroso-desmethyl-diltiazem to Table 2, and set an AI of 100 ng/day by read across from NNK. Now, not only nitroso-oxetines can be read a cross from NNK. Now it is the time to use NNK as a surrogate for all N-nitroso-N-methyl-N-alkylaryl NDSRIs and start moving away from the 18/26.5 ng/day AI for NDSRIs in general.
Dear Raphy,
That’s a good idea. I shared the nitrosamines whose AI is calculated by read-across from NNK.
Yosuke, you are correct. EMA and HC already used NNK as a surrogate to read across from for nitroso-desmethyl-citalopram.
On the other hand the read across from NNK to set a limit of 100 ng/day for nitroso-nornicotine is peculiar, in particular because its CPCA is a category 4. How is NNK an appropriate surrogate for nitroso-nornicotine?
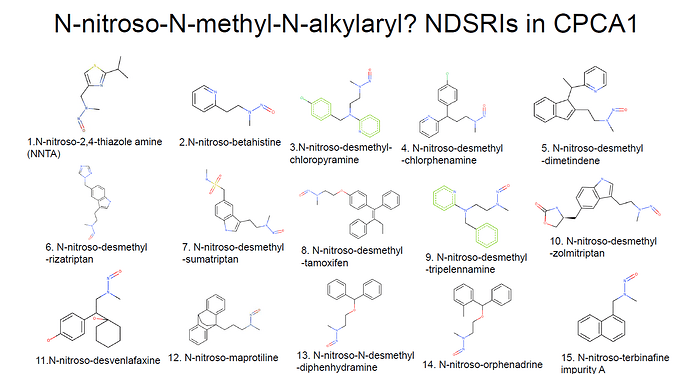
My proposal was to take all N-nitroso-N-methyl-N-alkylaryls that are all CPCA cat 1 and as a default (when there are no other considerations included) to read across for this entire class from NNK. I do not see how a nitroso-pyrrolidine analog can be read across from NNK.
I agree with you, Raphy. Nitroso-nornicotine looks unique in the figure. This may be related to the fact that it is considered a possible human carcinogen by the International Agency for Research on Cancer.
I collected nitrosamines like N-nitroso-N-methyl-N-alkylaryls in CPCA cat.1 from EMA Q&A appendix1. Should these 15 nitrosamines have 100ng/day?
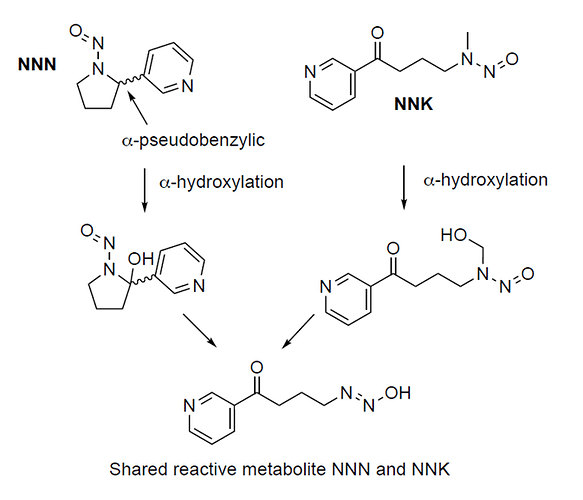
It has been described that alpha-hydroxylation of NNN in the 2-position (which has been found to be plausible despite steric hindrance) leads to the same diazohydroxide intermediate as alpha-hydroxylation of NNK on the N-methyl group. The TSNAs NNK and NNN thus have a common metabolite. This common pathway in NNK and NNN metabolism apparently leads to the same DNA adducts, whereas for the shared diazohydroxide metabolite mutagenic potential is established. As such it is predicted that the acceptable intake for NNN is comparable to the one for NNK. One can evaluate to use the Stoner 1998 study on NNN or the Rivenson 1988 study on NNK to set the AI of NNN, but value-wise this wouldn’t make a huge difference. Nonetheless one can make arguments why the application of CPCA would not necessarily mean an important safety issue for NNN and why balancing consistency with other interests is possible.
See also:
Fan, T., Sun, G., Zhao, L., Cui, X., & Zhong, R. (2019). Metabolic activation and carcinogenesis of tobacco-specific nitrosamine N’-nitrosonornicotine (NNN): A density function theory and molecular docking study. International Journal of Environmental Research and Public Health, 16(2), 178. Metabolic Activation and Carcinogenesis of Tobacco-Specific Nitrosamine N’-Nitrosonornicotine (NNN): A Density Function Theory and Molecular Docking Study; Hecht, S. S. (1998). Biochemistry, Biology, and Carcinogenicity of Tobacco-Specific N-Nitrosamines. Chemical Research in Toxicology, 11(6), 559–603. https://doi.org/10.1021/tx980005y; Hecht, S. S. (2008). Progress and Challenges in Selected Areas of Tobacco Carcinogenesis. Chemical Research in Toxicology, 21(1), 160–171. https://doi.org/10.1021/tx7002068; Ma, G., Yu, H., Xu, T., Wei, X., Chen, J., Lin, H., & Schüürmann, G. (2018). Computational Insight into the Activation Mechanism of Carcinogenic N’‑Nitrosonornicotine (NNN) Catalyzed by Cytochrome P450. Environmental Science & Technology, 52(20), 11838–11847. https://doi.org/10.1021/acs.est.8b02795; Ponting, D. J., Dobo, K. L., Kenyon, M. O., & Kalgutkar, A. S. (2022). Strategies for Assessing Acceptable Intakes for Novel N‑Nitrosamines Derived from Active Pharmaceutical Ingredients. Journal of Medicinal Chemistry, 65(23), 15584–15607. https://doi.org/10.1021/acs.jmedchem.2c01498; Stanfill, S. B., Hecht, S. S., Joerger, A. C., González, P. J., Maia, L. B., Rivas, M. G., Moura, J. J. G., Gupta, A. K., Le Brun, N. E., Crack, J. C., Hainaut, P., Sparacino-Watkins, C., Tyx, R. E., Pillai, S. D., Zaatari, G. S., Henley, S. J., Blount, B. C., Watson, C. H., Kaina, B., & Mehrotra, R. (2023). From cultivation to cancer: formation of N-nitrosamines and other carcinogens in smokeless tobacco and their mutagenic implications. Critical Reviews in Toxicology, 53(10), 658–701. https://doi.org/10.1080/10408444.2023.2264327; Thomas, R., Tennant, R. E., Oliveira, A. A. F., & Ponting, D. J. (2022). What Makes a Potent Nitrosamine? Statistical Validation of Expert-Derived Structure–Activity Relationships. Chemical Research in Toxicology, 35(11), 1997–2013; Wong, H. L., Murphy, S. E., & Hecht, S. S. (2005). Cytochrome P450 2A-Catalyzed Metabolic Activation of Structurally Similar Carcinogenic Nitrosamines: N‘-Nitrosonornicotine Enantiomers, N-Nitrosopiperidine, and N-Nitrosopyrrolidine. Chemical Research in Toxicology, 18(1), 61–69. https://doi.org/10.1021/tx0497696.
Standard application of the Bercu 2024 rule CPCA cat. 1-2 NDSRI MW > 200 Da → 150 ng/day as a rule in CPCA would also be a highly appreciated pragmatic first step, avoiding too much discussions on surrogate selection and avoiding in some cases the need for risk/benefit arguments to get acceptance of a readacross proposal, but instead upgrade automatically a lot of NDSRIs at once (while probably supporting easier implementation at non-NITWG member territories). I don’t understand why ongoing research to optimize the CPCA model prevents from already implementing this rule standard in the model now.
Dear Wybon,
I never imagined that the metabolites of the two nitrosamines would be the same. Thank you for sharing the information. NNK and NNN are both nitrosamines contained in tobacco smoke.
You read my mind, Yosuke-San ![]()
Very interesting the metabolite information. The question is not only the identity of the metabolite but the rate of metabolic activation. Does the alpha-hydroxylation of a nitroso-pyrrolidine occur as readily as the alpha-hydroxylation of a nitroso-N-methyl-N-alkylaryl? I would think not.
For the different options on NNN itself (not vis-a-vis other NNK analogues):
It is a bit complex because the rate of metabolic activation and predominance of 2’-hydroxylation of NNN is dependent on the stereochemistry of NNN (in principle (S)) and the specific CYP/tissue considered, but I believe that the (in vivo) metabolic relevance of 2’-hydroxylation of NNN linked to esophagus tumors (key for NNN) is sufficiently established, in vivo proof for pyridyloxobutyl (POB)–DNA adduct formation is available and detailed mechanistic investigations have been reported and linked with tumorigenesis, papers cited above show detailed insights in the subject. Since late nineties this metabolization route is considered at least a key route for NNN metabolic activation in vivo. The paper Hecht 2008 is a good place to start reading.
I think that regulator also wanted to be pragmatic, suitability to use Rivenson 1988 data on NNK is established, for Stoner 1998 robustness discussions would again be needed (though the most sensitive tumor site differ, the derivable AIs are not that different). (Nonetheless EMA/369136/2020 listed already NNN data in 2020).
Overall idea is that NNN shouldn’t be seen as the average N-nitroso-pyrrolidine/impact position on N-nitroso-pyrrolidine as a broader group, like put by Ponting et al:
the fact that NNN has a potent benzylic motif outweighs from a risk assessment perspective the fact that it also contains a pyrrolidine ring, which is a structural feature typically associated with lower carcinogenic potency
As it is a known limitation of the model of CPCA that synergies between structural features are not accounted for, the model cannot accurately predict the potency of NNN. CPCA takes into account the intrinsic lability of a (pseudo)benzylic C-H and thus its susceptibility to alpha-hydroxylation and not the likelihood of it leading to a more stabilized carbocation linked to the (pseudo)benzylic substitution or its link with further metabolization steps such as ring opening or susceptibility to alternative mechanisms. All (pseudo)benzyls are treated the same in CPCA. Unfortunately several CPCA features are looked at isolated from other CPCA features, whereas often synergy between functionalities can be expected.
Thomas et al. (above) have also used NNN as an example to explain that the key complexity in moving from expert assessment to statistically significant results, which CPCA seeks to address, is that any given nitrosamine is likely to be a member of multiple substructural categories. NNN has an isopropyl-like α-carbon, which is also benzylic and the different features may have a variety of effects that may variously increase or decrease potency. These may also mask the effect of each other, especially in the relatively small dataset that is available for nitrosamines. The deconvolution of these requires a statistical technique that is able to take dependencies in the data into account and precludes analysis of individual features in isolation.
The functionality masking effect and lack of functional synergy in CPCA is truly one of the bigger challenges for the QSAR advancing.
But wide extrapolation of features and their synergies can be difficult. The six-membered analogue N-nitrosoanabasine (NAB), which is of much lower potency doesn’t have the same type of mechanism. It has been suggested that the competition between alpha-hydroxylation and hepatic N-oxidation (as detoxification route) is different for NNN versus NAB, potentially linked to polarity differences. Additionally, it has been indicated that NAB and NNN might be metabolized by different CYP enzymes linked to their difference in structure:
Hecht, S. S. (1998). Biochemistry, Biology, and Carcinogenicity of Tobacco-Specific N-Nitrosamines. Chemical Research in Toxicology, 11(6), 559–603. https://doi.org/10.1021/tx980005y; Ponting, D. J., Dobo, K. L., Kenyon, M. O., & Kalgutkar, A. S. (2022). Strategies for Assessing Acceptable Intakes for Novel N‑Nitrosamines Derived from Active Pharmaceutical Ingredients. Journal of Medicinal Chemistry, 65(23), 15584–15607. https://doi.org/10.1021/acs.jmedchem.2c01498; Hecht, S. S., & Young, R. (1982). Regiospecificity in the metabolism of the homologous cyclic nitrosamines, N′-nitrosonornicotine and N′-nitrosoanabasine. Carcinogenesis (New York), 3(10), 1195–1199. https://doi.org/10.1093/carcin/3.10.1195; Thomas, R., Tennant, R. E., Oliveira, A. A. F., & Ponting, D. J. (2022). What Makes a Potent Nitrosamine? Statistical Validation of Expert-Derived Structure–Activity Relationships. Chemical Research in Toxicology, 35(11), 1997–2013; Chen, Y., Yang, Z., Zhou, Z., Liu, E. J., Luo, W., He, Z., Han, W., & Liu, Y. (2024). Metabolism-dependent mutagenicity of two structurally similar tobacco-specific nitrosamines (N-nitrosonornicotine and N-nitrosoanabasine) in human cells, partially different CYPs being activating enzymes. Toxicology (Amsterdam), 504, 153774. Redirecting.
this is amazing information…thank you very much Wybon for this free lesson ![]()
First I would like to thank Wybon for a very interesting and detailed discussion.
It is also interesting that the TD50 of NNK (0.0957 mg/kg/day, albeit the data is not considered robust) is almost the same as that of NNN (0.0999 mg/kg/day), and that may be explained by their both having the same mutagenic metabolic. What does surprise me though is that the metabolic hydroxylation of NNN at the benzylic position would be much more difficult than the hydroxylation on the methyl group of NNK, which would theoretically render NNN a much lower carcinogenic potency.
And ultimately, the question that I originally posed was why is NNN being read across from NNK, and I still do not see the logic in that.
Regarding the Bercu 2024 paper, the basic concept that we had behind this paper was that NDSRIs with MW > 200 should not be assessed the same as small dialkyl nitrosamines. The problem here is that NNN has a MW of 177 so Bercu 2024 would be relevant in this case. But I definitely do agree that implementation of the conclusion we had in the Bercu paper would be a great step forwarding for setting default limits for most NDSRIs.
Why is this in your opinion? It has been shown that the pseudobenzyl group as substituent on a ring system as form of possible steric hindrance is no issue for NNN. And mechanistically there is the intrinsic lability of a (pseudo)benzylic C-H and thus its good susceptibility to alpha-hydroxylation (the foundation for the activating feature in CPCA, rather than subsequent carbocation stabilisation). If you mean this from a perspective of the chance to identify enzyme matches from NNN vs. NNK based on substrate structure, there is a CYP match at oesophagus level.
Key flaw is indeed probably that the same metabolite and same order of magnitude of AI is not met by like for like tissue selectivity (Stoner 1998/Rivenson 1988) (linked to the difference in structure of NNN vs NNK affecting also CYP matches), using NNN data would have allowed to directly recognise the relevance of oesophagus tumor end point, matching also to where and how the effect of the shared metabolite materialises.
Thomas et. al evaluate NNN TD50 to be reliable enough (and EMA cited the data in 2020 report).
Thomas, R., Tennant, R. E., Oliveira, A. A. F., & Ponting, D. J. (2022). What Makes a Potent Nitrosamine? Statistical Validation of Expert-Derived Structure–Activity Relationships. Chemical Research in Toxicology, 35(11), 1997–2013.
Yes and it also not CPCA category 1-2, NNN is a separate discussion that came up here in connection with NNK readacross.
Not all CPCA category 1-2 NDSRIs would indeed be above 200 Da, but a relevant number is and as a first step this data-supported rule would really be a pragmatic way to advance some cases without needing difficult case-specific readacross discussions, allowing time to reprioritise efforts to generate muta/carci data on further gaps of the CPCA model. I highly appreciate the work in Bercu 2024, it should get valorised in CPCA by regulators without further unnecessary delay in my opinion.