Hello everyone ,
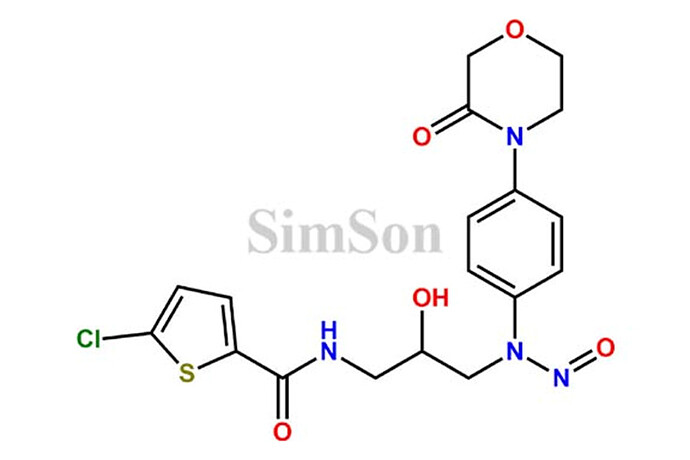
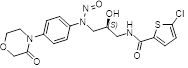
We have the challenge to separate the N-nitroso-rivaroxaban amide from API rivaroxaban with HPLC, which is the new NDSRI in appendix of EMA&FDA. Anyone have the experience regarding this impurity ? much thanks !!!
Dear Pu Zhao
We also working on separation of N-Nitroso Rivaroxaban amide impurity from Rivaroxaban Active peak. However it was very challenging to separate and still we working on with different column chemistry and diluents selections, So far not successful.
Dear Nitrosamine experts
We are looking forward your suggestions, how can convince agency regarding this impurity exemption?
if API ROS is not supporting to form this impurity.
Hi, it is very interesting to find the separation challenge. What’s the problem here? Is there a very similar polarity peak interfering or the whole reaction is very massy? I’d like to offer help, if possible.
Hi ,
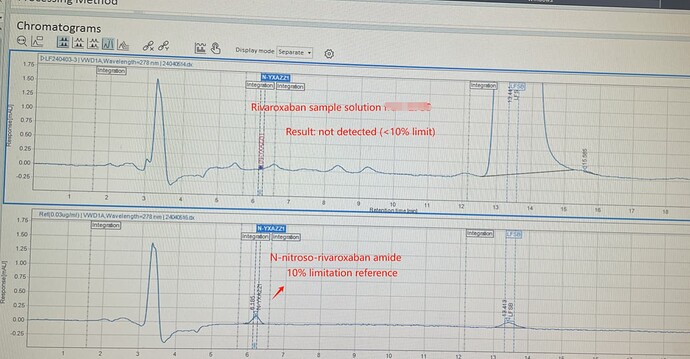
We have made some small progress in our research, separation method using normal phase chromatography, chiral columns and VWD detector .
The biggest problem is the sample solution is not completely dissolved and the sensitivity of the method (NP not suitable for LC-MS).
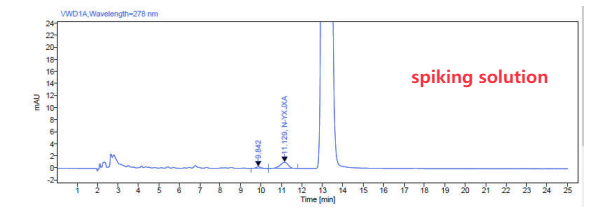
Chromatogram for information:
Hi,
Kindly use phenyl column. With 0.1% formic acid and methanol as mobile phase. We got better seperation of both impurities and Rivaroxaban peak. Got desired recovery on drug product.
Hello everyone ,
We have the similar challenge to separate the N-nitroso-rivaroxaban amide impurity from Rivaroxaban API using LC-MS, which is the new NDSRI in appendix of EMA&FDA. We have attempted several chromatographic columns, mobile phase compositions, literature articles, proposed conditions as available in this forum, but none of them could give us the positive outcome. It would be great if anyone can help us by sharing their experiences / suitable method conditions to separate Nitroso Rivaroxaban Amide Impurity from Rivaroxaban.
Thank you in advance.
Did u make it suitable for LC-MS, if yes, please share some chromatography conditions? Please!
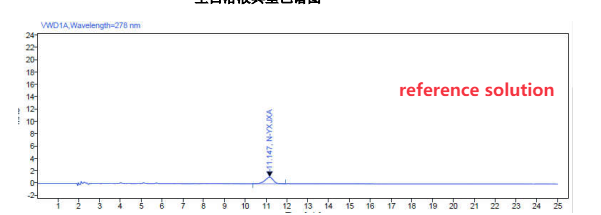
We have developed a HPLC-UV method , the limit for N-nitroso-rivaroxaban amide is 50ppm and the LOQ is 5ppm( which could be lower )
1. Chromatographic Conditions
| Parameter | Setting / Description |
|---|---|
| Column | ZORBAX SB-Phenyl, 4.6 × 150 mm, 3.5 µm |
| Mobile Phase A | 0.1% Phosphoric acid in water |
| Mobile Phase B | Methanol |
| Flow Rate | 0.8 mL/min |
| Column Temperature | 50°C |
| Detection Wavelength | 278 nm |
| Injection Volume | 25 µL |
| Diluent (Solvent) | 10% Acetonitrile |
| Special Configuration | A trap column is connected between the pump and the injector |
Elution Program (Isocratic)
| Time (min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|
| 0 | 52 | 48 |
| 25 | 52 | 48 |
2. Solution Preparation
Impurity Stock Solution
-
Procedure: Accurately weigh approximately 6 mg of N-Nitroso Rivaroxaban Amide impurity into a 20 mL volumetric flask. Dissolve and dilute to volume with Acetonitrile.
-
Concentration: 300 µg/mL.
Reference Stock Solution
-
Procedure: Pipette 0.2 mL of the Impurity Stock Solution into a 20 mL volumetric flask. Dissolve and dilute to volume with Acetonitrile.
-
Concentration: 3 µg/mL.
100% Reference Standard Solution
-
Procedure: Accurately pipette 1 mL of the Reference Stock Solution into a 10 mL volumetric flask. Dilute to volume with the Diluent (10% Acetonitrile) and mix well.
-
Concentration: 0.3 µg/mL.
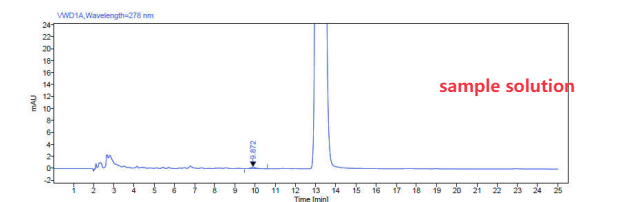
Test Solution (Sample Solution)
-
Procedure:
-
Accurately weigh approximately 60 mg of the sample into a 10 mL volumetric flask.
-
Add 1.0 mL of NMP (N-Methyl-2-pyrrolidone) and sonicate to dissolve.
-
Add 1 mL of Acetonitrile and mix.
-
Dilute to volume with water and mix well.
-
Let stand at room temperature for 10 minutes.
-
Filter before injection.
-
-
Concentration: 6 mg/mL.