Dear all experts,
I want to understand N-nitroso ruxolitinib chemistry and is this a possible N-nitroso impurity? If possible, what would be its acceptable intake? Should this impurity be tested?
Refer chemical name:
(R)-3-cyclopentyl-3-(4-(7-nitroso-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)propanenitrile
Similes input:
C1(CCCC1)C@@HN1N=CC(=C1)C=1C2=C(N=CN1)N(C=C2)N=O
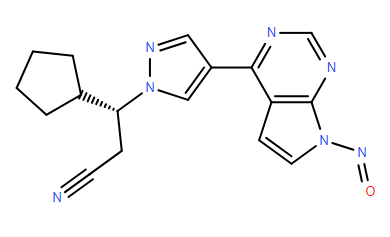
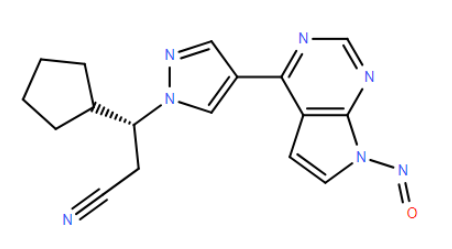
This structure comes from the non basic aromatic Nitrogen of Ruxolitinib, and according to Schlingemann "The Landscape of Potential Small and Drug Substance Related Nitrosamines in Pharmaceuticals (J. Pharm. Sci., 2022), it does not easily form nitrosamine or if it does this will not be part of cohort of concern. Relevant to the activity of this type of nitrosamines is “Drawing a Line - Where Might the Cohort of Concern End” Ponting and Foster, OPRD, 2023.
On the other hand, Melatonin type structures (e.g., N‐Nitroso -Melatonin - Synthesis, Chemical Properties, Potential Prodrug, Kirsch & de Groot, J. Pineal Res., 2009) easily form nitrosamines, therefore I think that the generation of nitroso- ruxolitinib (during manufacturing of the API and of the drug product and storage of DP) cannot be rulled out and should be tested for, if the standard is really available, as advertised.
2 Likes
Please do not forget the process impurities. I did a short search and found a possible dealkylation of alkyl pyrrole that could undergo nitrosation at the carbonyl compound… I am not an organic chemist, so I defer to the experts on this!!
3 Likes
Dear Mircea Florea,
Thank you for responding.
But it does not easily form nitrosamine or if it does this will not be part of cohort of concern. Also no potency score generated as per CPCA calculation.
Then why its is need to be tested?
Acceptable intakes of N-Nitrso ruxolitinib have also not been established by EMA, TGA, Health Canada, USFDA and ANVISA.
As you said the standard is really available, as advertised by other labs We have to be tested for
But in my experience many times commercially available standard mass numbers and other characteristic data of related impurities are not met.
The AI will be 1.5µg/day for a non CoC nitrosamine.
The usual risk assessment workflow applies.
In case of DS, it may be possible to rule out the nitrosamine formation considering the manufacturing process, contact with water, nitrite content of the water, acidic media… . Of course, as Naiffer mentioned, you should consider impurities and any vulnerable structure which may come into contact with nitrosating species during manufacturing.
In case of DP, it won’t be necessary to test for it if the formation of nitrosamine above AI can be ruled out considering worst case calculations based on the nitrite content of the excipients.
Are there doubts regarding the formation of N-nitroso-ruxolitinib? That means there is a risk, unless proving the contrary.
In my experience, often the advertised nitrosamine standards are not available, which sometimes means that it is not easy to be obtained. Then you can have a dialogue with the supplier about its synthesis and may obtain a proof that it was not possible to generate it in different conditions, e.g., according to EFPIA-Nitrosamines Quality Risk Management Workflows, Aug 2022.
2 Likes
yes, the impurity should be subjected to NDSRI content after categorizing into the slot. Please share the MS contents of the same.
I don’t have its characterization data right now, but earlier when I was doing theoretical evaluation, this impurity was not getting CPCA score. Have you evaluated this impurity?
This is clear in the guidance: “the potency categorization approach does not apply to N-nitrosamines where the N-nitroso group is within an aromatic ring (for example, nitrosated indole)”
3 Likes
could you please share the reason for not getting the CPCA score does the positioning of alpha and beta established with this.
1 Like
This is a N-nitroso impurity. However, this is not a nitrosamine. So, technically the nitrosamine related informaiton do not apply to this. You can do a surrogate study.
1 Like
Thank you madam, I expected this.
1 Like