@Chirag, @ASrinivasan and @Diego_HM Thanks for clarifying the doubt.
Is this difficulty in formation of Nitrosamide, applicable to all amides groups present in various molecules.
@JaideepJ it depends on electronic cloud present around, so you will need to justify case by case and along with this justification you have to present various trials at lab scale to conclude that even at favourable condition the said impurity is not possible in lab and hence cannot be present in Finish product also.
@Chirag, got it. Thanks
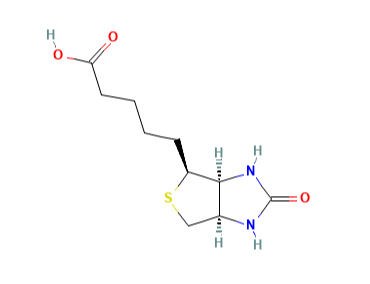
At the September meeting, the CMDh requested the NcWP to establish an acceptable intake of i.a. biotin. I wonder what decision will be made, given the lack of amine moieties in the biotin molecule.
@ASrinivasan @David @Yosukemino @Naiffer_Host @GENERAPHARM
Agencies make a clear distinction between N-Nitrosamines and N-Nitrosamides, e.g., EMA, Lessons learnt from presence of N-nitrosamine impurities in sartan medicines, and Nitrosamine guidance documents never mention Nitrosamides and the structures from which they derive.
As I understand, even if Nitrosamides are part of cohort of concern according ICH M7, as they are N-nitroso compounds, they are not required to be included in the Nitrosamine risk assessments.
Is this interpretation correct?
Nitrosamides are carcinogenic in many cases, as you mentioned and most definitely a cohort of concern. But the issue is not to mix them up with Nitrosamines, as the later needs activation by alpha hydroxylation to exert its carcinogenic activity, the former does not. Most of the nitrosamides do not need alpha activation and simple AMES Assay may predict their mutagenicity. But the main issue here is not to include them with nitrosamines. We are already suffering with almost 30% of pharmaceuticals being at risk. Including nitrosmides will possibly raise that significantly. May want to look at the white paper I published in Pharm. Tech. Nitrosamides–Should They Be Treated the Same as Nitrosamines?
Thank you for your reply.
In the article you mention it is stated “However, members of the pharmaceutical industry who have included amides of primary amines/alkylcarbamates/urea/guanidine in the same category with secondary and tertiary amines in their risk evaluation, need to keep in mind that the N-nitroso derivatives of the former are different from nitrosamines”
My point (and my question) is do you agree that according to Nitrosamine guidance documents Nitrosamides should not be included in risk assessments?
I understand that agencies preferred to simplify an already too complex crisis, however, I wonder why it is not stated clearly in these guidelines to not include Nitrosamides in the same category with Nitrosamines, given that ICH M7 does not make a distinction between them. This is what creates confusion.
Nitrosamides are not nitrosamines. All the guidance mention nitrosamines. In my opinion, from talking to a few agency members, the reason they did not mention nitrosamides is possibly because at that time, the institutional knowledge in the regulatory bodies was not enough to know where they were going with these N-nitroso compounds. If this was during my time in FDA and I were writing the FDA guidance, I would have used the term N-nitroso compounds and not nitrosamines.
Also, while FDA is denying, they really did not think of NDSRIs when they wrote this guidance. They were expecting people to analyze everything for about 6-7 nitrosamines. When they mentioned “Other Nitrosamines”, they were thinking of other small nitrosamines arising from reagents etc. not NDSRIs.
If you read the conclusion, I have stated that the jury is out on whether you should include nitrosamides in your risk evaluation. I think for now, we should follow the guidance and focus on nitrosamines. The nitrosamides can always be the next wave.
In short, your team needs to take a decision on this based on the science and what the agencies have said, if you want to include the nitrosamides.
@ASrinivasan
I appreciate your detailed response.
There are plenty of structures which can generate Nitrosamides, therefore it is a significant simplification if we can skip them in Risk assessments.
In the article published recently by the agencies, "Regulatory Experiences with Root Causes and Risk Factors for Nitrosamine Impurities in Pharmaceuticals (HC, EMA, FDA, ANVISA, HSA, J. Pharm. Sci., 2023), there is also no mention on Nitrosamides.
Although the EMA guideline does not clearly indicate nitrosamides, in September 2022 the CHMP adopted the CMDh conclusions addressed to the NcWP to determine the acceptable intake of N-nitroso-biotin.
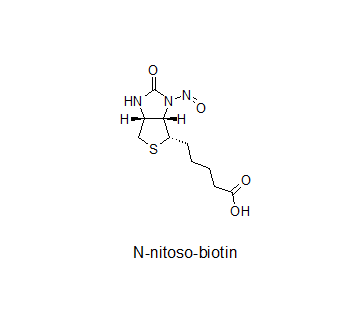
Biotin does not have nitrogen other than amide in its structure. Perhaps once the AI for biotin is established by the NcWP, we will know more about the agency’s approach to nitrosamides.
Nitroso-biotin is well known and a “nitrosamide”. I was told that there were sponsors who went to agencies with this, possibly without realizing that it is not technically a nitrosamine. Actually this was the reason, I wrote that “Horse of a Different Color” piece and also the white paper. I have seen several cases where the difference was not being understood and wanted to bring awareness of this issue. As stated before, jury is still out on this.
Would a nitrosamide potentially undergo activation through enzymatic α-hydroxylation if the necessary adjacent hydrogen is available? Is it possible some nitrosamides are mutagens by both direct action and by activation?
No, nitrosamides have a very different path of activation. They undergo a 1,3 sigmatropic shift and they are direct acting mutagen. Please read the paper I wrote it in a hurry and begged Pharm. Tech. because I started seeing people staring to control nitrosamides, etc. Nitrosamides–Should They Be Treated the Same as Nitrosamines?
It was also shared with FDA.
If you have more questions just write to me.
Now EMA nitrosamine Q&A appendix 1 includes Other N-nitroso-structures. Please check the following thread.
Thanks Yosukemino San, will wait for your opinions on the updates.
Thank you for your comment, Aloka. I feel that there is not enough information about other N-nitroso-structures. Nitrosamines are contaminated in pharmaceuticals because secondary amines are extremely reactive against nitrosating agents, whereas other N-nitroso-structures are just thought to be generated. Their reactivity is probably not as high as that of secondary amines. In addition, they may compete with the C-nitrosation reaction on the aromatic ring. The recall due to the contamination of other N-nitroso-structures has not been reported so far. Therefore, I would like to wait for the EMA to clarify why the AI of other N-nitroso-structures was published.
I am also worried about this. I had hoped that they will stop and resolve the secondary amine, teritiary amine nitrosation issue before doing anything else.
Hi all,
Internally, if I may say we also thought that indole rings tends to form more C-nitroso compounds than N-nitroso compounds. Interestingly, we found in preliminar studies both by means of a modified NAP test (Quite more % for N-nitroso) and we would need to go for method dev. and validation. I would say it would be very difficult to justify not evaluating them (nitrosated indoles at least), with this update.
I agree Diego, this is a message that the nitrosamides, Nitrosoguanidines, carbamates should be looked into at least for EMA. And we know that HC does whatever EMA does. So very soon, we may see this also in HC documents. FDA will procrastinate and let us hang ourselves with our own ropes, I guess. ![]()
![]()
We recently received a query from Health Canada regarding a compound containing a guanidine substructure, as opposed to an amine substructure, which may undergo nitrosation to form N-nitroso compounds. Health Canada acknowledged that while the impurity is not classified as a nitrosamine, it falls under the broader category of N-nitroso compounds, specifically N-nitroso-guanidines. They have recommended following the approach outlined in ICH M7 for the assessment and control of mutagenic impurities to establish an acceptable intake (AI).