In order to investigate the effect of nitrite and formaldehyde on the formation of NDMA in metformin preparations, we made preparations by spiking them with a certain amount. However, how should we interpret the fact that the amount of NDMA formation was significantly lower in tablets containing nitrite and formaldehyde together than in tablets containing only nitrite? This behavior does not indicate that formaldehyde is a catalyst for NDMA formation, unlike previous studies.
#metformin #NDMA Nitrite #Formaldehyde Excipients
It is not unexpected that formaldehyde condensates with metformin, leading to a degradation product that is not a good NDMA precursor. As such formaldehyde could be a nitrosation prevention additive here, just like antioxidant use in other cases, (but due to its reactivity it might indeed not be the best fit). This means that the xx ppm nitrite you spiked led to lack of robustness without formaldehyde and that part of the NDMA might have been formed during the preparation. Overall the method doesn’t seem to be robust in xx ppm nitrite.
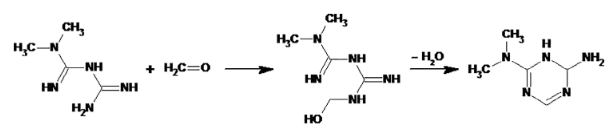
Mechanism of metformin formaldehyde scavenging:
https://doi.org/10.1002/tox.22982
Note that formaldehyde-catalysed nitrosation also has a higher pH optimum than classic nitrosation, so you have to be critical on your sample preparation when wanting to use this strategy:
Formaldehyde Catalysis of Nitrosamine Formation - New Scientific Knowledge & Development - Nitrosamines Exchange
It could also be that other ingredients in your product have a reactivity towards formaldehyde.
Note that in the the state-of-the-art there are different methods described for testing metformin preparations, also tackling robustness.
NDMA analytics in metformin products: Comparison of methods and pitfalls - ScienceDirect
(This is assuming NDMA can be formed from metformin itself, though in medicine detections a more prevalent root cause is dimethylamine contamination. In that case, metformin would still trap the possible catalyst formaldehyde possibly, rather than activating dimethylamine for formaldehyde-catalysed nitrosation if the pH allows it.)
Avoiding N-nitrosodimethylamine formation in metformin pharmaceuticals by limiting dimethylamine and nitrite - ScienceDirect
It has been known that the catalysis of nitrosation by nitrosamine is quite pH dependent and the kinetics is quite complicated. It had been agreed by stalwarts that formaldehyde, under certain conditions can catalyze nitrosation but again, we are seeing that many observations in solution phase are not applicable in solid phase. I think it is great that we continue the studies. But formaldehyde should not be considered as something that can deter nitrosation. Other than its inherent toxicity, its role in nitrosation is at best controversial.
For clarity, discussion reply was about the role of formaldehyde (or antioxidant) addition during sample preparation when investigating the robustness of sample preparation (linked to nitrosamine artefact formation) and has thus nothing to do with the toxicity of formaldehyde or the contamination of formaldehyde linked to some excipients and of course no proposal to add formaldehyde to medicine formulations as remediation. If formaldehyde catalyses the nitrosation during sample preparation, nitrosamine would go up with spiking of formaldehyde. If not, it would stay the same. But here there was a case observed where the nitrosamine went down with spiking of formaldehyde (indicative that the original sample preparation has a limitation robustness wise probably). I proposed to approach this from the angle that formaldehyde remains a reactive agent and has also a reactivity towards metformin coincidentally and can be indicative of the origin of lack of robustness of the original sample preparation. We are mostly focused on liquid phase here, formation during sample preparation. Of course this has to be approached with details on the levels, as RSD difference on sample on its own can be indicative of lack of robustness without clear formaldehyde role as well (reported as significant).
It has been raised in a critical discussion group:‘Impact of formaldehyde content in excipients on nitrosamine/NDSRI generation from vulnerable amines in FP’…
Your thought and any mechanistic publication reference would be greatly appreciated.
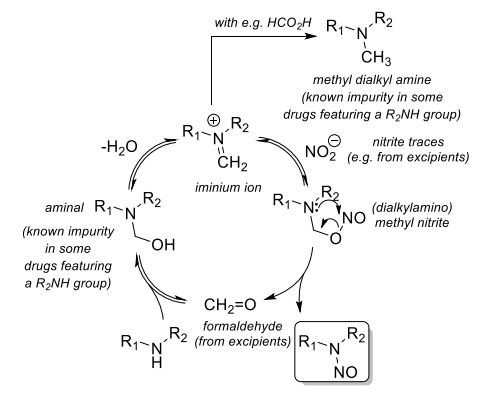
This was the mechanism that I had seen published previously.
As it loses water in the reaction then it was stated as being preferred in the solid state, compared to in liquids.
I’ll have to try and remember where I got this from…
Image is copied from Cioc 2023, Scheme 3
Thank you - when you had read so much you forget when you have seen things. I was trying to find the reference to give due credit.
My supervisor at NCI, Dr. Larry Keefer studied this a lot before he shifted to Nitric Oxide donors. Here is a paper,
Keefer LK, Roller PP. N-nitrosation by nitrite ion in neutral and basic medium. Science. 1973 Sep 28;181(4106):1245-7. doi: 10.1126/science.181.4106.1245. PMID: 4726444.