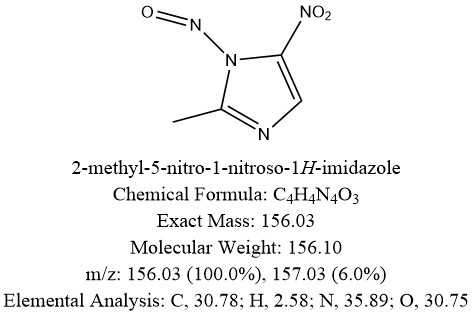
The possible nitrosamine impurities in metronidazole are shown in the figure below. It does not comply with CPCA. How should the limits be evaluated。
I believe the best option is to go with the surrogate approach. To reduce the error chances a software that uses the COMPUTATIONAL APPROACH and can deploy a sophisticated algorithm that measures the STRUCTURAL SIMILARITY of query Nitrosamine with the SURROGATES. The top surrogates should be presented along with their carcinogenicity TD50 values obtained from rodent carcinogenicity bioassays, experimental Ames mutagenicity test data, molecular weight, calculated or predicted LogP, and water solubility properties. With expert evaluation, appropriate surrogates are selected and once the TD 50 values are known can be used to derive an acceptable intake. We know of one such software QSAR Flex. A Webinar by the company will go live on January 9 and 12, and you can register for a date and time suitable using the below link, and copy-paste it in your browser window. Hope this will help you:
REGISTRATION LINK:
https://attendee.gotowebinar.com/rt/7410594456597570648