No guidance, but in the European context, it has come up a few times on the discussion table in meetings with Industry and the Agency since 2022. On the request to comment on the Fine 2023 paper, the NS OEG confirmed in December 2023 that international discussions continue and have stumbled on the molar thinking not been implemented on the experimental side as well:
MW adjustment is an issue that has cropped up ever since we started using some of the smaller nitrosamines as surrogates with the readacross approach and you know we have been considering it with other international regulators. But the principal argument against using these adjustments is that regulatory guidelines on toxicology studies and impurities, have always been considered based on weights and not other quantities and if we now start applying this MW adjustment on nitrosamines: just for the comparative assessment we would also have to think about perhaps extending it to Ames testing using molarity and in vivo studies as well (Ames: adjusting the reference doses to be applied to higher levels tested in case of higher molecular weight compound that is studied), and then higher levels of NDSRIs could need to be tested in Ames or in vivo mutagenicity testing, but we do have the CPCA: the readacross approach would probably rarely be used because we only have about 45 nitrosamine surrogates where we do have robust carcinogenicity data and it’s very rare that one of those could be used as a good comparator for an NDSRI.
→ Regulators perspective that if you use molecular weight corrections surrogate to NDSRI in CPCA and readacross, you also have to think about implementing the molecular weight correction in designing in vivo and vitro studies protocols, meaning a re-evaluation of the OECD 471 guidance on Ames testing? For example the Ames OECD 471 maximum plate concentration of 5000 µg/plate should then be defined as X µmole/plate (and thus be translated to µg/plate by multiplying with the molecular weight of the test compound). This means larger doses for NDSRIs with higher MW are to be applied (increasing testing cost, whereas background lawn clearance (toxicity) might anyway be seen for higher plate concentrations), whereas this also means an excellent purity of the NDSRIs will be needed to avoid misinterpretations, e.g. cf. Lijinsky recommendation:
Although it was possible - and was observed in some cases - that much larger quantities of nitrosamine than 1 mg could give rise to a number of revertants slightly above the minimum for significance, this cannot be considered acceptable procedure. The difficulty of excluding the presence of an undetectable trace of strongly mutagenic impurity in several milligrams of nitrosamine is so severe, that the significance of such marginal responses is moot. Therefore, the highest dose we used was 1 mg Redirecting.
The change in thinking in terms of molarity for mutagenicity evaluation from the perspective that 1 mole of a mutagenic function like nitrosamine (1 mole of mononitrosated compound) can lead to one mole of DNA alkylation should be done holistically, not only in readacross, but also in the toxicology study guidance: we fully think in terms of moles in mutagenicity or we don’t?
But then again, the nitrosamine saga isn’t over yet, shouldn’t we be more pragmatic?
As Industry commented to the EMA:
Over the years we’ve used milligram per kilogram body weight thinking in toxicology, but the toxicological data that was available was for a vast range of molecular weight of compounds. (So by studying classes of certain compounds, there was nothing wrong with thinking in mg/kg body weight because we had data for a certain class of compounds with sufficient variability in molecular weights and/or representative molecular weight for what we actually wanted to do readacross on). Nitrosamines as we know are different: we have only carcinogenicity data for very small nitrosamines (low MW, often dialkylnitrosamines), but we are studying higher MW nitrosamines (high MW) and are being confronted with the need to extrapolate low MW data to high MW applications, which we might not per se have seen when studying other mutagenicity cases (so the comparability can away not be fully restored). We just don’t have the data, but from understanding the mechanism that it is really stoichiometrically dependent mutagenesis that causes the carcinogenicity: the molecular weight argument is strong.
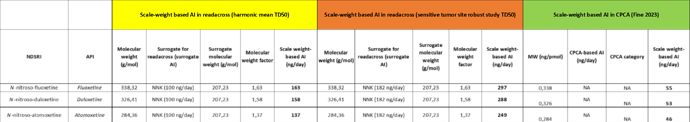
On a positive note: no MW correction capping discussions were confirmed by regulators, so I still believe that the caps I started to propose above can be arguable and visualise the thinking that MW corrections are needed to close important MW gaps for higher MW NDSRIs and can be verified with examples (e.g. NNK to NDMA readacross) to show the risks that the MW-corrected SAR-based AI is higher than the real AI. When the readacross is well justified and the nitrosamine is reasonably high in MW, an application of a capped MW correction as a next step should be possible?