Hey team, we need your expertise on this matter. We’ve encountered an excipient, Povidone K90, where the supplier has indicated potential traces of Diethanolamine up to 20 ppm and another excipient with nitrites up to 8 ppm. If both of these substances react, there’s a possibility of forming NDELA (the Acceptable Intake limit is 1900 ng/day). The APIs we’re using don’t pose any risks in terms of amines or nitrites. The risk seems to be solely from the excipients. How do we calculate the Acceptable Intake (AI) limit in the finished product? What would be the maximum daily dose? The amount of excipient with amine presence constitutes about 1.2% of the total formulation, and the one with nitrite risk is about 15% of the formulation. What steps should we take in this situation?
Hi Sarada,
i think that is a similar case with having vulnerable amines in your API which are going to be used in the formulation of the product. As in those cases the risk is concern NDMA, NDEA etc, in your case the risk is concerning the NDELA. So, the spec limit would be 1900ng/maximum daily dose of the API in your formula. Please, remember that the nitrite risk could be come from all the excipients you are using and not only from one. For example, Povidone K90 could contain traces of nitrites along with diethanolamine.
hope it helps
Hello,
I agree with Cristos: MDD based on API, as usual. Additionally, I would perform a worst case calculation for the limiting reagent, nitrite usually, but in this case could be DEA. If the result for full conversion exceeds the AI level, you may need to test for NDELA in the fresh drug product and stability samples to demonstrate that it does not accumulate during storage, following the pertinent guidelines.
I would also mention that there is literature indicating that the conversion of the nitrite in solid phase is plateauing at low levels. You may use this in your theoretical risk assessment, however, there is no guarantee that this will be accepted by the reviewer of health authorities.
I agree with @chrischar and @mflorea. Nitrites are the limiting agent. Of course, you should consider nitrites in the other excipients. When you calculate the amounts of possible nitrosamines, you can consider 100% conversion of nitrites first. What amount of drug products is taken a day at most? Here, the amount of amines is not so high, and the conversion rate is generally considered low.
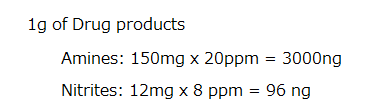
Remember that povidone can (does) contain an impurity
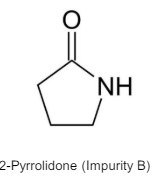
which can also presumably react with nitrite forming a nitrosamide. We tend not to worry about nitrosamides but it could compete for nitrite which would maybe be a good thing in this case.
My entire PhD was on nitrosamines in cosmetics, and guess what was the most prevalent one, NDELA. Both diethanol and triethanol amine (used in almost all cosmetics, lotions, creams etc.) produce NDELA very easily. I have been saying this since 2018 (when the valsartan and NDMA came out) that the oxides of nitrogen that the products are exposed to can form nitrosamines. I have seen this when I kept a cream exposed in a petridish and checked it for NDELA a few days later. So, please put a control in your Povidone, first for diethalnol amine and preferably test a few of your DP lots for NDELA. Absence of nitrite (which is also difficult to predict) is no reason to not add this control, as this nitrosamine is formed very easily.
@ASrinivasan was the NDELA from the triethanolamine itself, or from the diethanolamine impurities within it?
NDELA is originated by the diethanolamine impurity [max 0.5% in Trietaholamine (Trolamine) for pharmaceutical use (Ph.Eur.)].
Triethanolamine is quite sensitive to oxigen and it may be oxidized to diethanolamine (+acetaldehyde); this may increase the original amount of diethanolamine.
Generation of Maillard-type compounds from triethanolamine alone
However, this is relevant if triethanolamine is exposed to the air for a long time; oxidation does not occur in sealed containers (e.g. gels in aluminium tube).
Indeed, for an EFSA review of (re)activity of (N-nitroso-)2-pyrrolidone: Re‐evaluation of polyvinylpyrrolidone (E 1201) and polyvinylpolypyrrolidone (E 1202) as food additives and extension of use of polyvinylpyrrolidone (E 1201) | EFSA (europa.eu)
Whereas impurity B - 2-pyrrolidone is common for the povidone group, the amine contamination is process-specific and dependent on if a secondary or tertiary amine is used in the process and which one. Not all processes do use one, though process development literature does recommend diethanolamine or triethanolamine. Some manufacturers also have triethanolamine formate as an impurity instead of diethanolamine. It not being generalised explains why it is not part of EP or E-number specs for example.
Triethanolamine as well as diethanolamine gives NDELA when exposed to Nitric Oxide and air or higer oxides of nitrogen. In fact, most triethanol amine suppliers already have controls from NDELA. I recommend that if you are using trolamine (triethanolamine) in your formulation, look for NDELA.
It seems to me that it’s the Diethanolamine that is the limiting factor … Correct me if I am wrong ?
For 1g of Drug product :
Diethanolamine : 12 mg x 20 ppm = 240 ng
Nitrites : 150 mg x 8 ppm = 1200 ng
Yes, you are right. I am sorry for my mistake. 100% conversion of diethanolamine should be taken into consideration.