Hello everyone, I need to ask for your help with finding the most accesible method to detect and/or quantify small nitrosamines and NDSRIs. I’m from Chile, and there’s only 2 laboratories that have HPLC-MS/MS available to use.
I wanted to know if a conventional HPLC, with PDA and UV detector could do the job of doing a qualitative analysis, that means only detect the nitrosamines and not quantify them, so we can have an idea fo what we need to quantify.
I would think that it could wokr for small nitrosamines, but for NDSRIs a convetional HPLC-MS could do the same? A qualitative analysis that could show if there’s presence or not, so we can know what we need to quantify.
I really appreciate your help in advanced!
Hi Soledad!
HPLC-UV analysis is not sensitive and selective enough for testing or screening for nitrosamines at ppb levels. In certain cases, even LC-MS-MS techniques face challenges regarding sensitivity or due to interferences (e.g., NDMA - DMF), or contaminations (e.g., NDBA - filters).
It can be sometimes used in the case of drug products destined for advanced cancer treatment following ICH Q3A/B limits, which are much higher.
Instead, a thorough theoretical risk assessment can save time and analytical resources, indicating what you need to test for.
Hi @mflorea !
Thank you so much for your response! Based on what you said, a conventional HPLC-UV or HPLC-PDA would not be enough even for just detect the NAs?
Yes, I’m doing de risk assessment first to evaluate the risk of presence of nitrosamines, however, here in Chile, It’s possible that a new version of the Finished Porducts Specification Guide is going to be published. If it happens, the detection and quantification of small nitrosaminas, at least, should be included in the Finished Product Specification, so it would be ideal if we could do at least the detection so we don’t have to send all of our products to an external entity.
Again thank you so much for your help!!
Unfortunately, it is not possible to detect NAs at the AI levels required by HPLC-UV
I’d like to add that indeed, you will find methodologies for HPLC-UV, such as this one here (But making it clear that it is embedded within the context of the early methodologies):
Determination of NDMA and NDEA in SARTAN drug substances by HPLC/UV
On October 27, 2020, EMA/503522/2020 already stated: “Ensure that OMCLs have adequate resources to deal with the requested workload and are equipped with modern instrumentation for analyzing mutagenic impurities at trace levels (liquid chromatography–mass spectrometry and gas chromatography–mass spectrometry, etc.).”
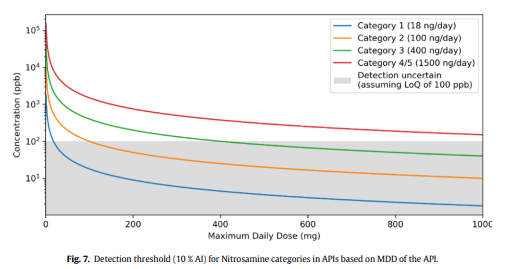
What we’ve seen over the past few years of discussion is that we are facing increasingly challenging limits. As an example, I reference this work (Revisiting the Landscape of Potential Small and Drug Substance Related Nitrosamines in Pharmaceuticals), where the authors state:
“To prove the absence of an NDSRI, analytical methods are required to have an LOQ at 10 % of the content limit derived from the NDSRI’s acceptable intake and the API’s maximum daily dose. Quantification of an NDSRI has been estimated to be routinely achievable at levels down to 50-100 ppb. However, in the case of NDSRIs for which control in categories 1-3 is required by the CPCA, this may not be sufficient, as the low acceptable intakes - in combination with even moderate allowed daily API doses - may result in LOQ requirements beyond technical feasibility. The analytical challenge may be exacerbated if the NDSRI co-elutes with the API due to high physicochemical similarity, or by the lack of robust reference standards.”
Hi,
On the regulatory aspect I would really wonder if general nitrosamines would be included as part of the Finished Product Specification Guide for other compounds apart from sartans. That surely deviates from the concept of ICH Q9 and the risk evaluation to actually determine what nitrosamine makes sense (if any) in a particular drug product formulation.
Practically speaking, there would be for sure a bottleneck around analytical capabilities. I would try to challenge, either by industry associations or other forum if that happens.
Finally, on the analytical side, you could use HPLC-UV to detect nitrosamines, but not at the LOQs needed currently for pharmaceuticals. Therefore, of no avail.
