Kindly suggest how to calculate Total nitrosamine impurities in Sitagliptin and Metformin HCl Tablets (FDC).
How to add this limit in specification part???
Dear Sandip,
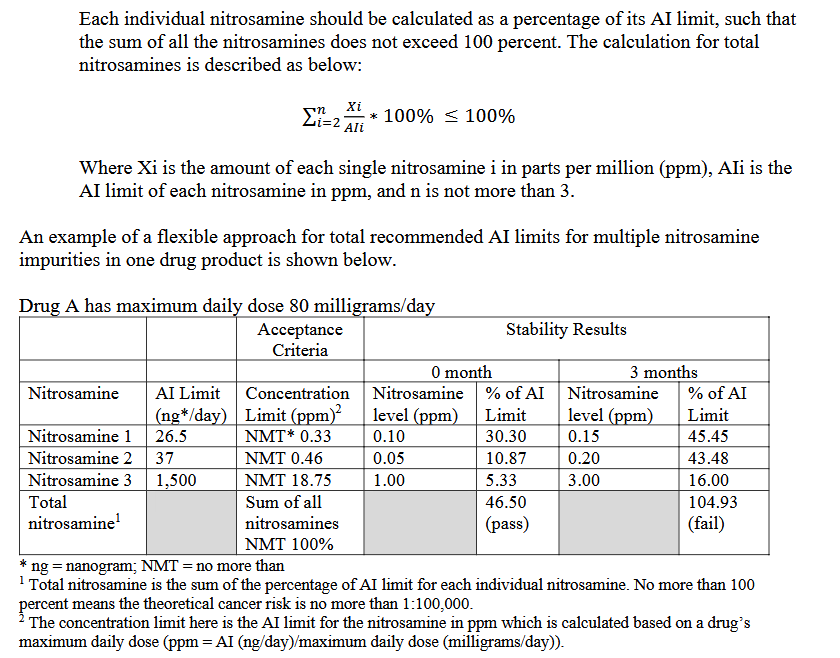
EP and FDA propose the ‘‘flexible’’ approach in the corresponding guidelines. The total nitrosamine content is described as ‘‘Sum of all nitrosamines NMT %’’, as it is show below (Guidance of industry (FDA))
The only difference is that in FDA guideline this could not be used if you have more than three nitrosamines in the product while in the EP guideline is not presented this limitation
hope this helps
Christos
Dear Chrischar,
Thanks for above information.
I need more clarity regarding above calculation is applicable for fixed dose combination contain 2 or 3 nitrosamine impurities or Not??
i.e one API contain NDMA and second API contain NTTP, in this case the above calculation is applicable or not??
Dear Sandip,
yes, the ‘‘flexible’’ approach is applicable for final product generally, there is no specific limitation in none of the guidelines.
thank you
Christos
Dear Sandip,
You can choose either fixed or flexible approach based on your product assessment.
However it is essential to kept Total nitrosamines in the drug product specification if it warrants.
Dear Chrischar,
Can we go for individual limit in specification for both Nitrosamine impurities??
Dear Sandip,
i am not sure what exactly you are asking.
As it is presented in the example of FDA guideline, in the specs indicidual limits for each nitrosamine are presented along with the limit for the total, which is presented as percentage.
Could you please share an example for better understanding?
thanx
Christos
Dear Chrischar,
For example, in case of Sita + Met IR Tabs contains two nitrosamine impurities, NMDA in Metformin HCl and NTTP in Sitagliptin API, in these case what is the specification limit for these two impurities.
Both API contains single Nitrosamine Impurities, so we can give Total of these two or Single impurities limit for these two Nitrosamine impurities??
Dear Sandip,
i think that the example of FDA is very close to your case.
Lets simplify this and lets suppose that for your product the max. daily dose for Metformin is 2000mg and for Sitagliptin is 100mg.
Then for each one nitrosamine the spec would be:
METFORMIN: 96ng/2000mg = 0.048ppm
SITAGLIPTIN: 100ng/100mg=1ppm
TOTAL NITROSAMINE: Sum of all nitrosamines NMT 100%.
So, if in one batch you have e.g. 0.025ppm metformin and 0.2ppm sitagliptin, then the SUM would be: (0.025/0.048+0.2/1)*100 = (0.52+0.2)*100=72<100. So, your batch is consider OK.
But in case e.g. the content of the NAs are
Metformin: 0.05ppm and Sitagliptin: 0.5ppm (both inside the spec. limits), then the total would be
Sum: (0.025/0.048 + 0.5/1)*100=102>100. This batch would fail even the values for the individual NAs are inside the spec. limit.
hope now is clear
Christos