Hi @Naiffer, this is btw a very interesting topic.
Are we referring to synthetic peptides/oligonucleotides or are we talking on purely biologics generated by cells? If it´s the case, where and what are we looking for? It´s related to single use bags, filling processes, extractable and leachable?
Thanks for giving me some light!
@Frabaneda Those are all good questions… we open this category exactly to get an understanding: Is there a risk? if so, Where exactly the risk lies?.. I think we could agree that the risk if any is surrounding the finished product (packaging, inorganic excipients, solvents, etc).
What has been your experience? Including my colleague @Annu in the discussion.
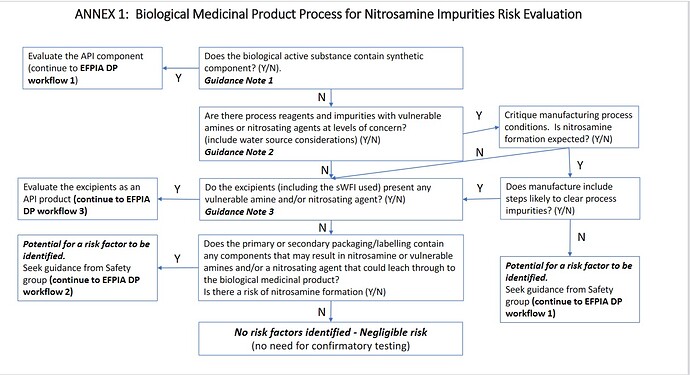
Hi, @Frabaneda. I have never treated biological products so far, however I found the biological part of EFPIA workflow, it was linked from the slide presented by Dr. Urquhart. As @Naiffer_Host pointed out, the part which have risks seems corresponded. In addition that, EFPIA concluded most of biological products were considered as very low risk. What do you think about it?
Hi @Yosukemino Thanks for the slide.
My view is similar to the EFPIA when talking about pure biologics. Said that, I have doubts when we´re talking on synthetic compounds (peptides, oligonucleotides…) were probably a risk assessment should be done to identify potential risks like some of the reagents and solvents.
And I agree with @Naiffer_Host around the packaging. After discuss with some experts on Extractable and Leachable they are considering that the next “big thing” could potentially be nitrosamine for all pharmaceutical products after the filling and packaging. So, probably it is an open exploratory way and in fact we´re working on this to see how to perform some analytics using LC-MS
Thank you @Frabaneda. I agree with you. And I guess EFPIA considered biological products as low risk with considering synthetic compounds you mentioned. Of course risk assessment is important.
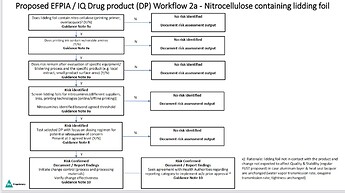
And thank you for sharing the risk of packaging. EFPIA Flow has the packaging part, which targets Nitrocellulose.
@DAB also introduce interesting webinar.
I expect the nitrosamine contamination risk from Pkg Materials are not high in many cases when considering manufacturing and storage conditions… Does anyone have comments?
I agree with you about peptides and oligos. In traditional biologics also, it is possible to get nitrosamines from various sources!
Though chances are less.