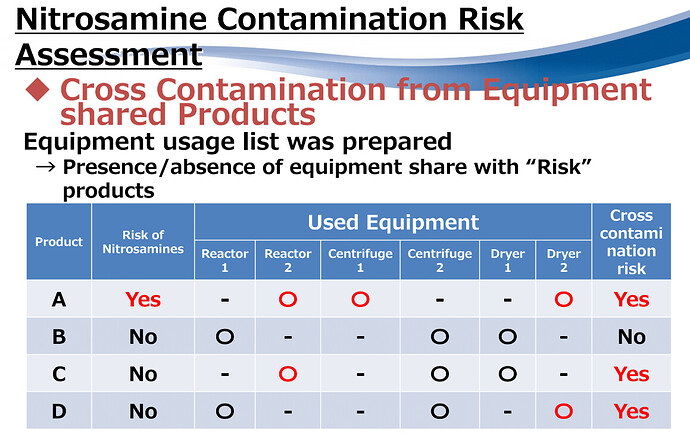
At the recently celebrated MHLW/PMDA - USP Workshop, Nitrosamines Impurities were a core topic of discussion. Japanese manufacturer Daito shared some of their strategies for Risk Assessment. Are you considering risk on ‘shared’ equipment on multi-product manufacturing?
Hello Naiffer @Naiffer_Host
This is a very important topic that also requires attention from the GMP perspective, thanks for sharing .
Appreciate more Insights in this matter.
If product A is contaminated with impurity amounts of nitrosamine, manufacturing under GMP and general cleaning validation can prevent cross contamination enough, I think. This is why, where and how much amount of nitrosamine is contaminated in the manufacturing process of product A is important. And if high risk of contamination is concerned, some additional treats such as cleaning validation targeting nitrosamine, confirmatory testing or unsharing the equipment are considered. Anyway, in many cases, cross contamination from other products do not look a serious problem on shared equipment, because they are cleaned appropriately.