The publication “Nitrosamines crisis in pharmaceuticals - insights on toxicological implications, root causes and risk assessment: A systematic review” is readily available online and accessible with an open access format.
Overall, the article provides an extensive examination of the nitrosamines crisis within the pharmaceutical industry.
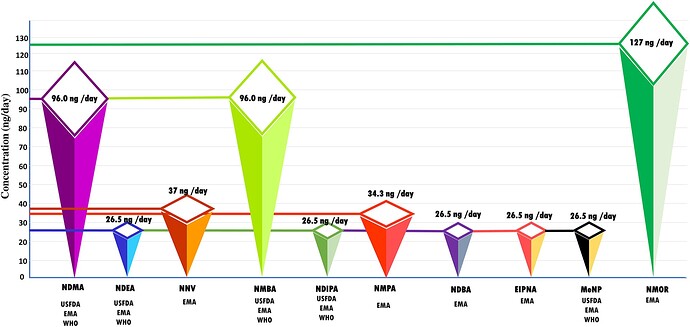
• The article explores the ramifications of nitrosamines from a toxicological standpoint, delving into the most recent updates regarding the toxicity profiles of N-nitroso compounds.
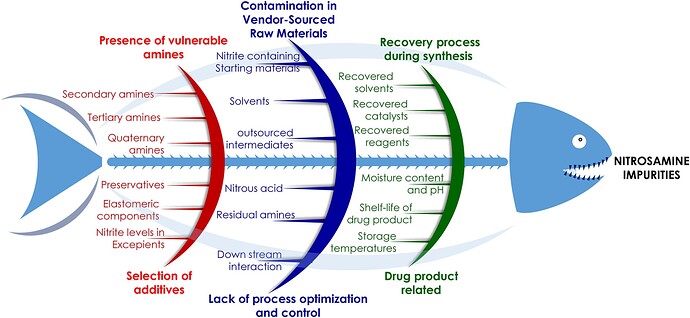
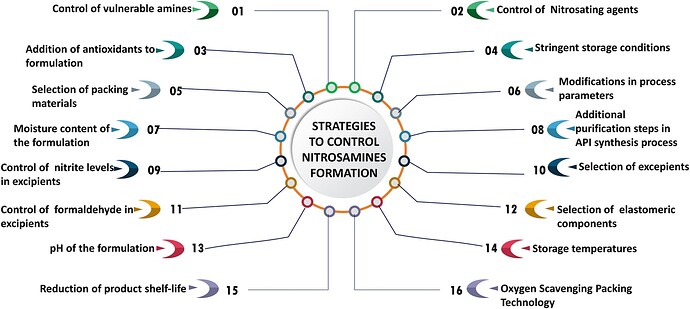
• Additionally, the publication thoroughly discusses the root causes behind the nitrosamines crisis, alongside comprehensive risk assessment strategies and mitigation approaches specifically designed for pharmaceutical manufacturers.
• The authors shed light on the utilization of Gas Chromatography (GC) and Liquid Chromatography (LC) methods for the precise detection and quantification of N-nitroso compounds present in pharmaceutical products.
• An underlying emphasis throughout the article revolves around the imperative need to develop pharmaceutical products free from nitrosamine contaminants, ensuring adherence to regulatory standards.
Within this review, it’s important to underscore the authors’ mention of significant challenges encountered during the development of analytical methods.
These challenges stem from issues such as inadequate ionization of analytes and interference from matrices.
Specifically, in the context of NDSRIs, complexities arise due to molecular weights, intricate structures, and the scarcity of readily available standards.
Furthermore, the authors have mentioned our coummunity: “To address the nitrosamine crisis, the USP is hosting a virtual platform called the “nitrosamines exchange” (…). Additionally, USP has established a “nitrosamine analytical hub” to provide updates on novel analytical methods for testing nitrosamines.”
It’s an evolving subject that matures daily, evolving through each new discovery and discussion.
What caught your attention the most in the article?