Dear all,
Do you think it would be likely that nitrosamines were formed from tertiary amines in azabicycles? For example, as the bicyclo structure in solifenacin:
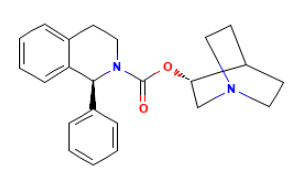
Thank so much for the comments!
Dear all,
Do you think it would be likely that nitrosamines were formed from tertiary amines in azabicycles? For example, as the bicyclo structure in solifenacin:

Thank so much for the comments!
Theoretically, under the right conditions, Hydrolysis of Carbamate ester and subsequent nitrosation of the secondary amine.
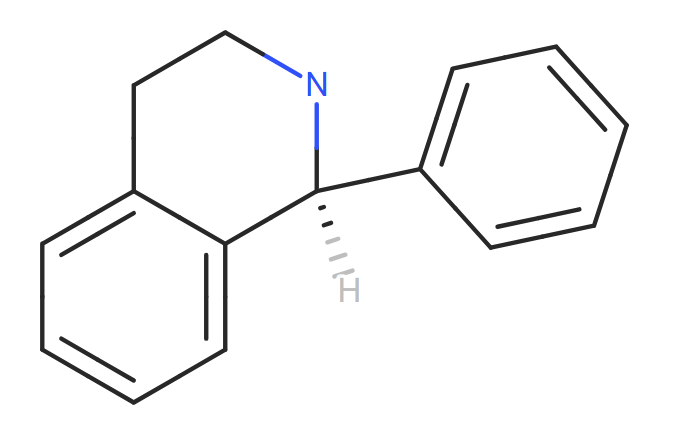
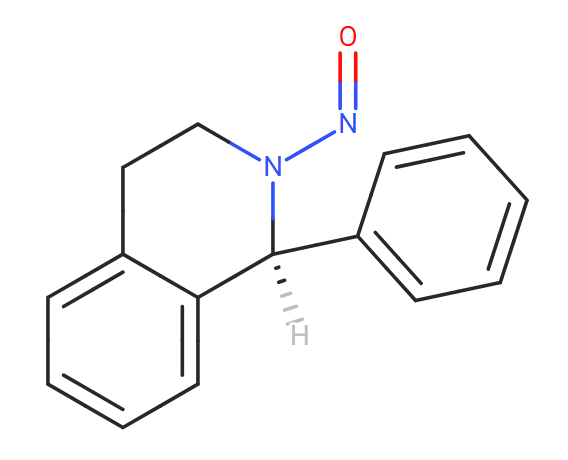
Thanks so much Naiffer!
And in your opinion, can we assume that the aza biciclo is not a problem?
I suppose that in the hypothetical case that this cyclic was opened (nitrosative cleavage), it would form an aldehyde and a secondary amine that would in turn react intramolecularly, and therefore would not generate a nitrosamine…
Again, thanks so much for your feedback ![]()
Regarding to tertiary amine in azabicycles, you can support your risk analysys with paper by Smith and Loeppky in which inertion of tertiary bridged nitrogen of Quinuclidine for nitrosation was shown.
https://pubs.acs.org/doi/10.1021/ja00981a021
Thanks so much for you reply and paper Rafa! It really helps!!