Hello colleagues. I wanted to ask if any of you have knowledge about the formation of nitrosamines in solution at pH 7. Thank you very much
Hi, welcome in this forum.
Theoretically the nitrosaction reaction at pH 7 should be disadvantaged.
" It is acknowledged that pH 3 to 4 is considered optimal for nitrosation with nitrous acid and at higher pH the nitrosation reaction becomes much less likely. If pH > 7 for the process and product then the risk for nitrosation of amines with trace nitrite is considered negligible. There is some evidence that pH 5 to 7 can also be low risk for nitrosation but needs to be considered on a case by case basis."
Ref. Workflows for Quality risk management of nitrosamine risks in medicines
However, in other cases the reaction of nitrosation is nearly independent of the pH; see e.g.
Kinetics of the Nitrosation of Pyrrolidine and Proline
Summarizing, at pH = 7 the risk is much lower than at acidic pH, but it cannot be excluded at all.
kind regards
Dear @e.blejman,
I think the following paper will help you understand the behavior of nitrite in several conditions. You can consider the pKa of amines, temperature, and the amount of nitrite and amines other than pH.
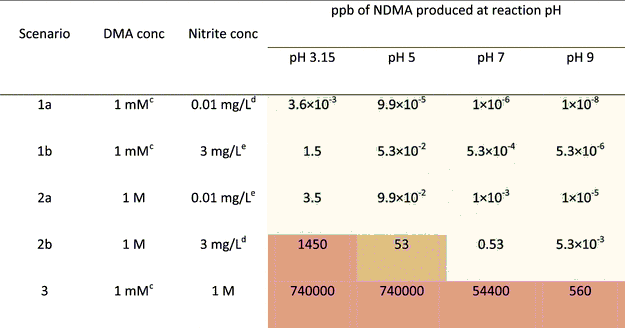
it is possible in the presence of some catalysts, eg.,
Also,
A discussion on the topic can be also found in

In Larry Keefer’s lab at National Cancer Institute, where I worked for many years, we saw a lot of nitrosation at neutral pH due to presence of trace compounds like formaldehyde. In Dr. Loeppky’s lab, we have seen nitrosation which happens even in neutral pH when some cosmetics with amines in them were exposed to nitric oxide and oxygen. We considered N2O3 as the active species.


