Pfizer is restricting the shipments of and eventually plans to recall its antidepressant Amoxan (amoxapine) in Japan after detecting cancer-causing impurities. When I get further information, I will post it.
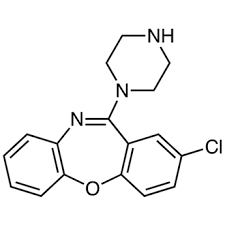
Amoxapine
Pfizer is restricting the shipments of and eventually plans to recall its antidepressant Amoxan (amoxapine) in Japan after detecting cancer-causing impurities. When I get further information, I will post it.

Amoxapine
Adding some relevant impurity information based on ChemIDPlus (NIH):
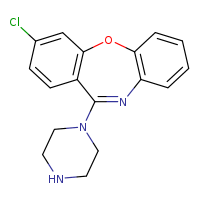
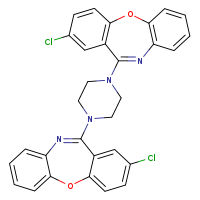
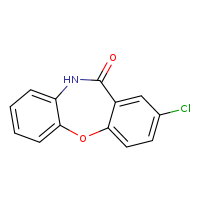
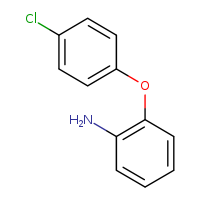
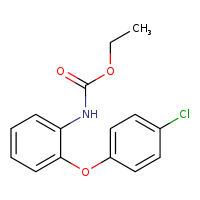
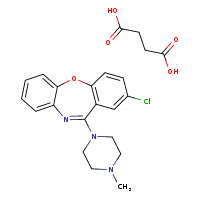
According to the Japanese Ministry of Health, Labor and Welfare, nitrosamine impurity seems nitrosated API.
According to Pfizer Japan, the acceptable intake of N-nitroso-amoxapine was calculated as 153 ng/day.
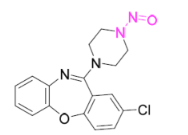
N-nitroso-amoxapine
They referred to the article by Krista L. Dobo et al. (2021) " Practical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N -Nitrosamine Impurities ". And they reviewed the TD50 of eight nitrosamines with a piperazine structure in Structural Group 10 for the read-across approach. The TD50 ranged from 0.140 to 34.6 mg/kg/day. And they decided on 1,2,6-trimethyl-4-nitrosopiperazine as the surrogate because the TD50 was reliable and the lowest, except for 1-Methyl-4-nitrosopiperazine. The TD50 of 1-Methyl-4-nitrosopiperazine was considered unreliable because the TD50 was derived from a single-dose study in which there was a 100% incidence of olfactory carcinomas. There is no reliable indication of the lower-bound estimate of carcinogenic potency.
They concluded the TD50 of Structural Group 10(= 153 ng/day) was acceptable. And NIHS in Japan announced the approach was reasonable.