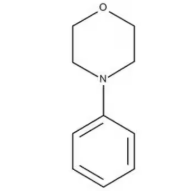
Can the bond between Morpholine and Benzene be broken and hence morpholine nitrosated to N-nitroso Morpholine?
I believe that the answer in this case is, in case of doubt, do a Nitrosation assay.
In my opinion, the risks comes more from phenylmorpholine containing certain amounts of morpholine as impurity which can be nitrosated rather than direct nitrosation of phenylmorpholine
5 Likes
Is it possible for the morpholine ring to open? if yes which bond will be more susceptible to break?
Thank you!
we need @conudel to answer the organic chemistry here!! thanks Raphy
Could happen, but it is unlikely, since 6-member rings are quite stable. You would require very harsh reaction conditions to be able to open a morpholine ring. I would not worry about it too much unless mentioned harsh conditions are used
1 Like