what is the probability for a primary amine to form a nitroso group, is it active for such reaction?
Primary amines form instable diazonium ion, not nitrosamines.
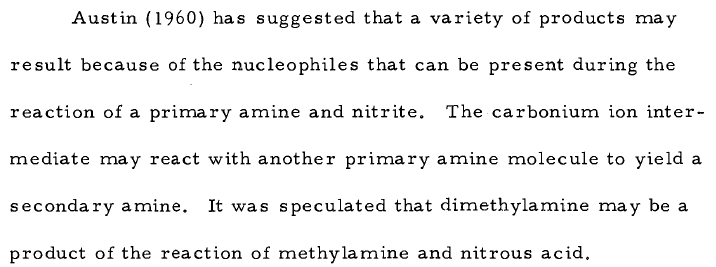
Theoretically, the diazonium ion may alkylate the original (primary) amine to form a secondary amine and this one may form nitrosamines.
However this mechanism is quite improbable and requires an excess of nitrite.
An update on the current status and prospects of nitrosation pathways and possible root causes of nitrosamine formation in various pharmaceuticals
No it cannot form.
Primary amines react with nitrosating reagents to form short lived diazonium ions which further decomposes to give molecular nitrogen.
Primary amine unable to form nitrosamine but dueing manufacturing of Primary amine, if secondary or tertiary will be present as an impurity than chances are there. So better to review impurity profile of any primary amine molecule.
I have found few possibilities for the formation of nitrosamines due to “Primary amines” in the publication titled “FORMATION OF HETEROCYCLIC N-NITROSAMINES FROM THE REACTION OF NITRITE AND SELECTED PRIMARY DIAMINES AND AMINO ACIDS”.
This is very old literature dated June 25, 1974 from Food Science and Technology.
There are many others case studies describing the conditions under which primary amine is susceptible to form nitrosamine impurities.
Requesting experts to share their views on this and further approach for the risk assessment with respect to the presence of primary amines.
Full publication is attached herewith for your reference.
WARTHESENJOSEPH1975 (1).pdf (1.1 MB)
Further, you can access the full thesis and article through the following links:
I believe nobody will argue that primary amines will not under transformation, but the reaction condition to carry out are atypical in a pharmaceutical process. That’s why is commonly acceptable to be a no risk factor.
As it is discussed above, the presence of the secondary amines as impurities of the primary amines (coming from the manufacturing processes) is the risk to be considered.