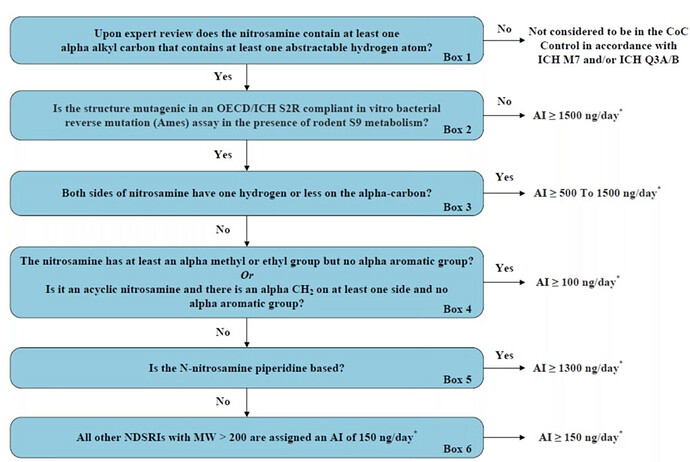
At recent meetings between Industry and both FDA and EMA, it was proposed an approach to define temporary acceptable intake limits for nitrosamine drug related substance related impurities (NDSRIs) with a MW > 200 Da
The proposal took features and principles from several publications that try to quantify and define risk based on the chemical characteristics of the nitrosamine structure.
What do you think? How feasible is the adoption of these models?
5 Likes
Hi @Naiffer_Host …This resembles similar to EFPIA proposed approach.
Dear @Naiffer_Host, the presented flowchart marks a significant advancement in our understanding and approach to addressing the issue of N-nitrosamine impurities in drug products.
The research conducted by Dobo et al. (2022) sheds light on the toxicity levels associated with different N-nitrosamine structural groups. The finding that a considerable number of these groups exhibit TD50s close to or surpassing 1.5 mg/kg/day raises concerns regarding their potential adverse effects on human health. Moreover, the classification of 76% of N-nitrosamine impurities into a group exceeding the precautionary default limit of 18 ng/day emphasizes the importance of setting appropriate limits to ensure patient safety.
The complex nature of N-nitrosamine impurities necessitates continuous research and technological advancements to refine our understanding and detection methods further. I have no doubt that the presented flowchart serves as a valuable tool in the ongoing efforts to establish more accurate and comprehensive limits for N-nitrosamines in drug products.
2 Likes
Hi Naiffer,
I understand that the general approach is currently not accepted by regulators (indeed no guideline mention this) even as interim limits, as well as any suggestion of using molecular weight into the equation. It is something that is needed, a standarized framework with a clear pathway to set up limits, but I think we are still a bit far away on having regulatory acceptance of this kind of approaches.
What of course may be accepted is the individual evaluation in a case by case with a Weight of evidence approach, for example proposing your particular piperidine based nitrosamine to use a 1300 ng/day, etc.
What is needed to get this acceptance? I think can be another question to put into the table. For example, starting from the top a modified methodology that put the Ames test back into consider it reliable results for regulators when negative, etc. The same until the bottom.
2 Likes
I second your thoughts @Diego_HM. I am not sure about regulatory acceptance but as per my experience this approach does not fit to all nitrosamines structures. I experienced few challenges.
Coming to application wise or WoE building, this is really invaluable criteria!
But the most interesting about this approach is, about the areas where we still feel little uncertainty about application of them to nitrosamines are given confident weightage in this approach (alpha methyl and Ames assay)!
2 Likes
As I mentioned in my post, this is a proposed approach presented by industry. It does incorporate version or classification from the Dobbo approach.
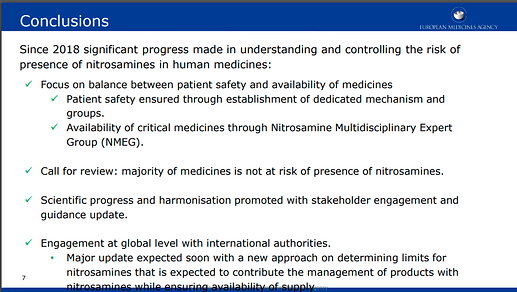
My understanding is that the European agency mentioned that the Q&A document will be revised by end of summer and we will see a major update related to NDSRIs, also a version of this framework might be in the update. Several members are optimistic about the upcoming update
3 Likes
Indeed, we are all eagerly awaiting the regulatory position on the matters we have been discussing, @Naiffer_Host.
In light of the ongoing discussions and research surrounding nitrosamine impurities, it is encouraging to see the collaborative efforts among regulatory bodies, industry experts, and scientific communities worldwide.
As we await the regulatory position, it is important for us, stakeholders, to continue sharing knowledge, conducting research, and implementing proactive measures to mitigate the presence of nitrosamine impurities. Collaboration between regulatory agencies, pharmaceutical manufacturers, and analytical laboratories will be crucial in establishing robust strategies for risk assessment, detection, and control of nitrosamines.
2 Likes
Hi Naiffer thanks for your answer. Indeed are proposals where as you mentioned maybe part of the decision tree would be accepted but not all as there are still gaps in some parts (e.g.: Ames test).
One additional question that came to my mind after your feedback on EMA Q&A. What does “Temporally” in the title means under your perspective? A temporally limit as for example the 1 year or 3 year EMA defines or limits for the long term lets say until further uniquevocal evidence is generated (e.g: in-vivo data). I ask b/c there is of course a difference between the two possible concepts.
According to the slide, a major update is expected soon with a new approach on determining limits for nitrosamines that is expected to contribute the management of products with nitrosamines while ensuring availability of supply. This is what we are looking forward to!!
4 Likes
Thanks for sharing! I was looking for the publication date and found it here (05/07/2023):
2 Likes
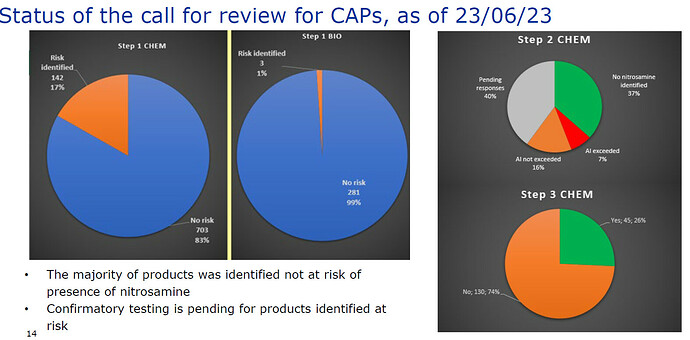
I found 40% of step 2 was pending at 23/06/23…