Discussing nitrosamines (NAs) poses several challenges, which might seem repetitive to mention, do you agree?
Amidst extensive discussions concerning NDSRIs, the focus of this publication is directed towards low-molecular-weight NAs and an analytical approach that provides a more dependable tool for routine quality control.
The study (A robust analytical method for simultaneous quantification of 13 low-molecular-weight N-Nitrosamines in various pharmaceuticals based on solid phase extraction and liquid chromatography coupled to high-resolution mass spectrometry ) aimed to create and validate an analytical method using SPE-LC-HRMS for simultaneously detecting 14 low-molecular-weight NAs across a wide range of APIs and DPs (24 APIs prone to NA contamination and on diverse DPs containing these APIs, including formulations with combinations of two or more APIs).
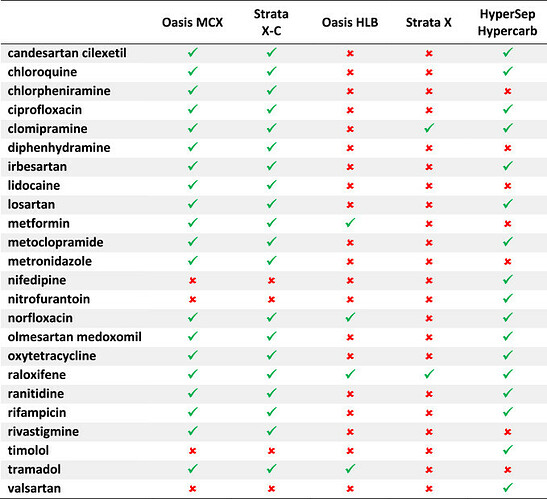
Various SPE sorbents were tested to prevent APIs from being present in the sample. Oasis HLB and Strata X, known for their effectiveness in retaining various substances, showed limited API retention when methanol was used for elution. Oasis HLB retained four APIs, while Strata X retained only two.
In contrast, HyperSep Hypercarb, recognized for NA determination in drinking water, exhibited better results. When eluted with methanol, it retained 17 out of the 24 selected APIs, including polar compounds.
However, Oasis MCX and Strata X-C displayed improved retention, holding onto most of the selected APIs (20 out of 24) when eluted with a solution of 5% H3PO4 in methanol. Despite being weak acids, Valsartan, nifedipine, and nitrofurantoin were well-retained due to these sorbents’ combined reversed-phase and strong cation exchange mechanisms.
Oasis MCX and Strata X-C were chosen for further assessment in NA extraction processes based on their superior retention capabilities.
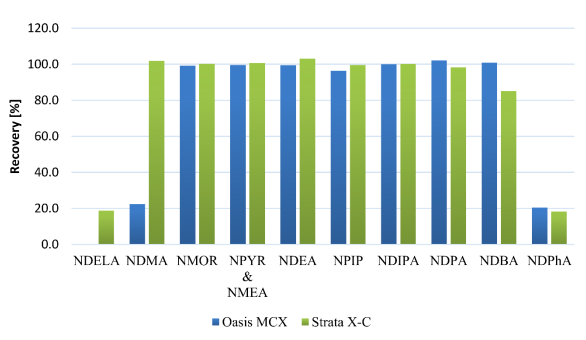
Strata X-C showed superior performance by retaining nearly all NAs during sample loading and eluting them easily with acidified methanol. Recoveries for nine out of eleven NAs exceeded 80%, including the highly polar analyte NDELA. Conversely, Oasis MCX failed to retain NDELA and showed inadequate retention for NDMA, resulting in only around 20% recovery. NDPhA’s strong retention on both cartridges led to lower recoveries due to π-π and hydrophobic interactions. Consequently, Strata X-C was selected for NA extraction from various DPs based on these outcomes.
The article provides further details on method validation. For instance:
“The robustness of the method was assessed by varying the quantity of DP at the QCAI level (quality control samples at acceptable intake limit). Different amounts of DP (equivalent to 3%, 6%, and 9% of the maximum daily dose (MDD) of API) were pre-spiked with QCAI solution and prepared via SPE extraction in triplicate. Robustness was determined based on accuracy (%), with the acceptance criterion set at 70–130%”.
Therefore, given the questions raised by regulators and stakeholders about the performance characteristics of MS-based analytical procedures, I’d like to initiate a discussion on this topic.