Hi, I need help from Organic Chemistry experts, this compound is related impurity with spec of 1.0% in my drug product. Can this compound be easily nitrosated? Thank you
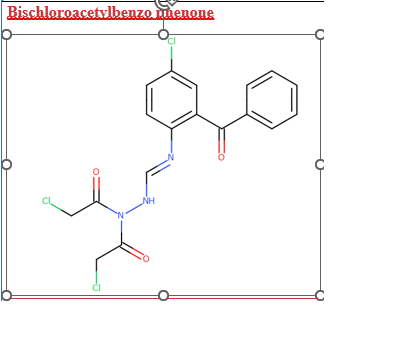
Hi, I need help from Organic Chemistry experts, this compound is related impurity with spec of 1.0% in my drug product. Can this compound be easily nitrosated? Thank you

Prima facie, I don’t see a vulnerable dialkyl amine in this molecule. So the nitrosation seems to be difficult.
Are you aware of any of its degradation products? I believe, they too would not be susceptible to N-nitrosation.
Thank you Muzaffar
Actually this is known identified impurity in drug product (Estazolam). I updated the post title to give more clarity
This is structure of Estazolam
That is an interesting molecule. Of course, the nitrosation where the N-H bond is would not for a nitrosamine. This is like a substitued hydrazone (or azene like compound?). Therefore, the evaluation of this possible nitroso compound is out of FDA Nitrosamine guideline. However, could be considered as part of ICH M7.
In particular, there is quite some steric hindrance there. Also that molecule not sure how stable it is. Prior to a nitrosation an hydrolysis may be a more realistic possiblity or a break of the N-N bond perhaps, that are the single bonds where the lowest energy is needed for a breakage to happen. Where the N-H bond is none of the elements on the alpha position are sp3.
Isn’t this impurity genotoxic by itself ? a spec of 1% appears to be very high.
It includes indeed a trisubstituted hydrazine and an imine moiety. The later could hydrolyze and generate a primary amine which does not form a stable nitrosamine. I do not see any risk of nitrosation of this impurity.
Thank you so much Deigo, Mflorea
My concern actually about the NH in the middle, If it possibly forming nitrosamine without hydrolysis of the molecule.
The spec level is set at 1% as this identified impurity in DP with MDD of 2 mg/ day
That would not be a nitrosamine, in the acceptance of the current guidance documents
Thank you for providing the additional information. My initial comment was if you were aware of any of the secondary degradation products of this degradation impurity. I completely agree with @mflorea that a secondary degradation of this impurity would result in a primary amine which does not form a stable nitrosamine. Further, as stated by @Diego_HM nitrosation of this parent impurity is difficult and would not result in something which you have to worry about. Only the dialkyl N-nitrosamines which are susceptible to alpha-hydroxylation are within the cohort of concern. My assessment indicates that this impurity as such or its degradation products (if any) would not yield a CoC N-nitrosamine.
Hope this helps ![]()
I would certainly be surprised if a N-nitrosoderivative of that impurity was stable
2-(N -Chloroacetyl-N -chloroacetylhydrazonomethyl)amino-5-chlorobenzophenone or Bischloroacetylbenzo
phenone is limited to 0.1% in the USP monograph of Estazolam
In Inxight Drugs (2-(N-CHLOROACETYL-N-CHLOROACETYLHYDRAZONOMETHYL)AMINO-5-CHLOROBENZOPHENONE), the structure of this impurity is represented as:
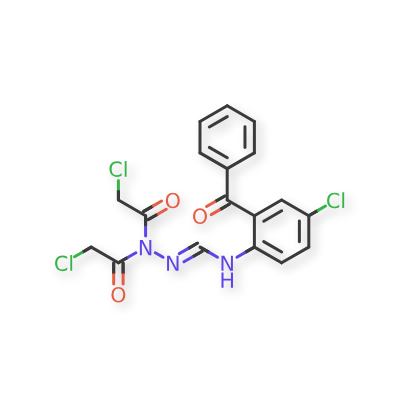
Several hypothetical nitroso derivatives would be possible, but no nitrosamines
Thank you so much for confirming the nitrosation possibility. You are right the specs in Estazolam raw material is 0.1%. For drug product we are following ICHQ3b based on MDD the client set the specs in drug product at 1% or 50 ug